Atmospheric sciences
- NOx (or NOx) refers to the sum of NO and NO2. [1] [2]
- NOy (or NOy) refers to the sum of NOx and all oxidized atmospheric odd-nitrogen species (e.g. the sum of NOx, HNO3, HNO2, etc.)
- NOz (or NOz) = NOy − NOx
- Mixed Oxides of Nitrogen ("MON"): solutions of nitric oxide in dinitrogen tetroxide/nitrogen dioxide.
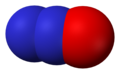
- Nitric oxide , NO
- Nitrogen dioxide , NO2
- Nitrous oxide , N2O
- Dinitrogen trioxide , N2O3
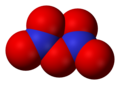
- Dinitrogen tetroxide , N2O4
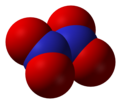
- Dinitrogen pentoxide , N2O5
- Trinitramide , N4O6