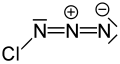 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name Chlorine azide | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChemSpider | |||
PubChem CID | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| ClN3 | |||
| Molar mass | 77.4731 g/mol | ||
| Appearance | Yellow-orange liquid; colorless gas | ||
| Melting point | −100 °C (−148 °F; 173 K) | ||
| Boiling point | −15 °C (5 °F; 258 K) | ||
| Solubility | Soluble[ vague ] in butane, pentane, benzene, methanol, ethanol, diethyl ether, acetone, chloroform, carbon tetrachloride, and carbon disulfide; slightly soluble in water | ||
| Structure | |||
| orthorhombic | |||
| Cmc 21, No. 36 [1] | |||
| Explosive data | |||
| Shock sensitivity | Extreme | ||
| Friction sensitivity | Extreme | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards | Extremely sensitive explosive | ||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Related compounds | Hydrazoic acid Fluorine azide Bromine azide Iodine azide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Chlorine azide ( Cl N 3) is an inorganic compound that was discovered in 1908 by Friedrich Raschig. [2] Concentrated ClN3 is notoriously unstable and may spontaneously detonate at any temperature. [3]


