In organic chemistry, a carbonyl group is a functional group with the formula C=O, composed of a carbon atom double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a carbonyl group is often referred to as a carbonyl compound.
In chemistry, azide is a linear, polyatomic anion with the formula N−3 and structure −N=N+=N−. It is the conjugate base of hydrazoic acid HN3. Organic azides are organic compounds with the formula RN3, containing the azide functional group. The dominant application of azides is as a propellant in air bags.
Phosphorus trifluoride (formula PF3), is a colorless and odorless gas. It is highly toxic and reacts slowly with water. Its main use is as a ligand in metal complexes. As a ligand, it parallels carbon monoxide in metal carbonyls, and indeed its toxicity is due to its binding with the iron in blood hemoglobin in a similar way to carbon monoxide.
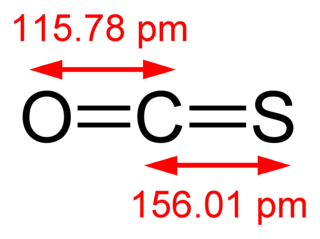
Carbonyl sulfide is the chemical compound with the linear formula OCS. It is a colorless flammable gas with an unpleasant odor. It is a linear molecule consisting of a carbonyl double bonded to a sulfur atom. Carbonyl sulfide can be considered to be intermediate between carbon dioxide and carbon disulfide, both of which are valence isoelectronic with it.

Iron pentacarbonyl, also known as iron carbonyl, is the compound with formula Fe(CO)5. Under standard conditions Fe(CO)5 is a free-flowing, straw-colored liquid with a pungent odour. Older samples appear darker. This compound is a common precursor to diverse iron compounds, including many that are useful in small scale organic synthesis.

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel tetracarbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometallic complexes.

In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually restricted to small ligands such as pseudohalides or to ligands that are specifically designed to link two metals.
Carbonyl fluoride is a chemical compound with the formula COF2. It is a carbon oxohalide. This gas, like its analog phosgene, is colourless and highly toxic. The molecule is planar with C2v symmetry, bond lengths of 1.174 Å (C=O) and 1.312 Å (C–F), and an F–C–F bond angle of 108.0°.
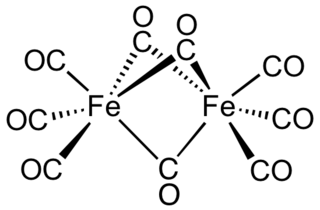
Diiron nonacarbonyl is an organometallic compound with the formula Fe2(CO)9. This metal carbonyl is an important reagent in organometallic chemistry and of occasional use in organic synthesis. It is a more reactive source of Fe(0) than Fe(CO)5. This micaceous orange solid is virtually insoluble in all common solvents.

Tungsten hexacarbonyl (also called tungsten carbonyl) is an organometallic compound with the formula W(CO)6. This complex gave rise to the first example of a dihydrogen complex.
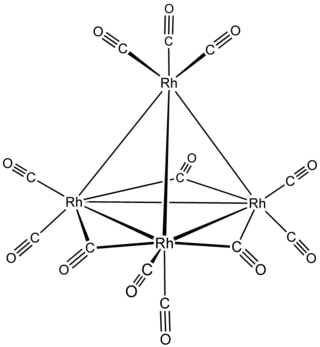
Tetrarhodium dodecacarbonyl is the chemical compound with the formula Rh4(CO)12. This dark-red crystalline solid is the smallest binary rhodium carbonyl that can be handled as a solid under ambient conditions. It is used as a catalyst in organic synthesis.
Barium azide is an inorganic azide with the formula Ba(N3)2. It is a barium salt of hydrazoic acid. Like most azides, it is explosive. It is less sensitive to mechanical shock than lead azide.

Silicon tetraazide is a thermally unstable binary compound of silicon and nitrogen with a nitrogen content of 85.7%. This high-energy compound combusts spontaneously and can only be studied in a solution. A further coordination to a six-fold coordinated structure such as a hexaazidosilicate ion [Si(N3)6]2− or as an adduct with bicationic ligands Si(N3)4·L2 will result in relatively stable, crystalline solids that can be handled at room temperature.

Bromine azide is an explosive inorganic compound with the formula BrN3. It has been described as a crystal or a red liquid at room temperature. It is extremely sensitive to small variations in temperature and pressure, with explosions occurring at Δp ≥ 0.05 Torr and also upon crystallization, thus extreme caution must be observed when working with this chemical.
Nickel compounds are chemical compounds containing the element nickel which is a member of the group 10 of the periodic table. Most compounds in the group have an oxidation state of +2. Nickel is classified as a transition metal with nickel(II) having much chemical behaviour in common with iron(II) and cobalt(II). Many salts of nickel(II) are isomorphous with salts of magnesium due to the ionic radii of the cations being almost the same. Nickel forms many coordination complexes. Nickel tetracarbonyl was the first pure metal carbonyl produced, and is unusual in its volatility. Metalloproteins containing nickel are found in biological systems.

Tetrabutylammonium is a quaternary ammonium cation with the formula [N(C4H9)4]+, also denoted [NBu4]+. It is used in the research laboratory to prepare lipophilic salts of inorganic anions. Relative to tetraethylammonium derivatives, tetrabutylammonium salts are more lipophilic but crystallize less readily.
Jaqueline Kiplinger is an American inorganic chemist who specializes in organometallic actinide chemistry. Over the course of her career, she has done extensive work with fluorocarbons and actinides. She is currently a Fellow of the Materials Synthesis and Integrated Devices group in the Materials Physics and Applications Division of Los Alamos National Laboratory (LANL). Her current research interests are focused on the development of chemistry for the United States’ national defense and energy needs.

Caesium azide or cesium azide is an inorganic compound of caesium and nitrogen. It is a salt of azide with the formula CsN3.

Boron triazide, also known as triazidoborane, is a thermally unstable compound of boron and nitrogen with a nitrogen content of 92.1 %. Formally, it is the triazido derivative of borane and is a covalent inorganic azide. The high-energy compound, which has the propensity to undergo spontaneous explosive decomposition, was first described in 1954 by Egon Wiberg and Horst Michaud of the University of Munich.

Sulfuryl diazide or sulfuryl azide is a chemical compound with the molecular formula SO2(N3)2. It was first described in the 1920s when its reactions with benzene and p-xylene were studied by Theodor Curtius and Karl Friedrich Schmidt. The compound is reported as having "exceedingly explosive, unpredictable properties" and "in many cases very violent explosions occurred without any apparent reason".