
Lead(II) azide Pb(N3)2 is an inorganic compound. More so than other azides, it is explosive. It is used in detonators to initiate secondary explosives. In a commercially usable form, it is a white to buff powder.
In chemistry, azide is a linear, polyatomic anion with the formula N−3 and structure −N=N+=N−. It is the conjugate base of hydrazoic acid HN3. Organic azides are organic compounds with the formula RN3, containing the azide functional group. The dominant application of azides is as a propellant in air bags.

Sodium azide is an inorganic compound with the formula NaN3. This colorless salt is the gas-forming component in some car airbag systems. It is used for the preparation of other azide compounds. It is an ionic substance, is highly soluble in water, and is very acutely poisonous.

Hydrazoic acid, also known as hydrogen azide, azic acid or azoimide, is a compound with the chemical formula HN3. It is a colorless, volatile, and explosive liquid at room temperature and pressure. It is a compound of nitrogen and hydrogen, and is therefore a pnictogen hydride. The oxidation state of the nitrogen atoms in hydrazoic acid is fractional and is -1/3. It was first isolated in 1890 by Theodor Curtius. The acid has few applications, but its conjugate base, the azide ion, is useful in specialized processes.
The azide-alkyne Huisgen cycloaddition is a 1,3-dipolar cycloaddition between an azide and a terminal or internal alkyne to give a 1,2,3-triazole. Rolf Huisgen was the first to understand the scope of this organic reaction. American chemist Karl Barry Sharpless has referred to this cycloaddition as "the cream of the crop" of click chemistry and "the premier example of a click reaction".
Sodium oxide is a chemical compound with the formula Na2O. It is used in ceramics and glasses. It is a white solid but the compound is rarely encountered. Instead "sodium oxide" is used to describe components of various materials such as glasses and fertilizers which contain oxides that include sodium and other elements.
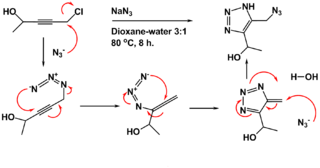
The Banert cascade is an organic reaction in which an NH-1,2,3-triazole is prepared from a propargyl halide or sulfate and sodium azide in a dioxane- water mixture at elevated temperatures. It is named after Klaus Banert, who first reported the process in 1989. This cascade reaction is unusual because it consists of two consecutive rearrangement reactions.
A monatomic ion (also called simple ion) is an ion consisting of exactly one atom. If, instead of being monatomic, an ion contains more than one atom, even if these are of the same element, it is called a polyatomic ion. For example, calcium carbonate consists of the monatomic cation Ca2+ and the polyatomic anion CO2−
3; both pentazenium (N+5) and azide (N−3) are polyatomic as well.
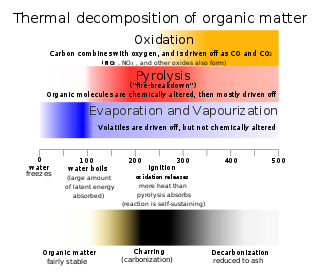
Thermal decomposition is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic as heat is required to break chemical bonds in the compound undergoing decomposition. If decomposition is sufficiently exothermic, a positive feedback loop is created producing thermal runaway and possibly an explosion or other chemical reaction.

The Curtius rearrangement, first defined by Theodor Curtius in 1885, is the thermal decomposition of an acyl azide to an isocyanate with loss of nitrogen gas. The isocyanate then undergoes attack by a variety of nucleophiles such as water, alcohols and amines, to yield a primary amine, carbamate or urea derivative respectively. Several reviews have been published.

Silver azide is the chemical compound with the formula AgN3. It is a silver(I) salt of hydrazoic acid. It forms a colorless crystals. Like most azides, it is a primary explosive.
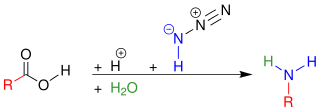
In organic chemistry, the Schmidt reaction is an organic reaction in which an azide reacts with a carbonyl derivative, usually an aldehyde, ketone, or carboxylic acid, under acidic conditions to give an amine or amide, with expulsion of nitrogen. It is named after Karl Friedrich Schmidt (1887–1971), who first reported it in 1924 by successfully converting benzophenone and hydrazoic acid to benzanilide. The intramolecular reaction was not reported until 1991 but has become important in the synthesis of natural products. The reaction is effective with carboxylic acids to give amines (above), and with ketones to give amides (below).
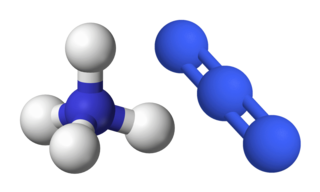
Ammonium azide is the chemical compound with the formula [NH4]N3, being the salt of ammonia and hydrazoic acid. Like other inorganic azides, this colourless crystalline salt is a powerful explosive, although it has a remarkably low sensitivity. [NH4]N3 is physiologically active and inhalation of small amounts causes headaches and palpitations. It was first obtained by Theodor Curtius in 1890, along with other azides.

Potassium azide is the inorganic compound having the formula KN3. It is a white, water-soluble salt. It is used as a reagent in the laboratory.
Barium azide is an inorganic azide with the formula Ba(N3)2. It is a barium salt of hydrazoic acid. Like most azides, it is explosive. It is less sensitive to mechanical shock than lead azide.

Chlorine azide is an inorganic compound that was discovered in 1908 by Friedrich Raschig. Concentrated ClN3 is notoriously unstable and may spontaneously detonate at any temperature.

Methyl azide is an organic compound with the formula CH3N3. It is a white solid and it is the simplest organic azide.

Bromine azide is an explosive inorganic compound with the formula BrN3. It has been described as a crystal or a red liquid at room temperature. It is extremely sensitive to small variations in temperature and pressure, with explosions occurring at Δp ≥ 0.05 Torr and also upon crystallization, thus extreme caution must be observed when working with this chemical.

Fluorine azide or triazadienyl fluoride is a yellow green gas composed of nitrogen and fluorine with formula FN3. Its properties resemble those of ClN3, BrN3, and IN3. The bond between the fluorine atom and the nitrogen is very weak, leading to this substance being very unstable and prone to explosion. Calculations show the F–N–N angle to be around 102° with a straight line of 3 nitrogen atoms.

Iodine azide is an explosive inorganic compound, which in ordinary conditions is a yellow solid. Formally, it is an inter-pseudohalogen.














