
Arsenic is a chemical element with the symbol As and the atomic number 33. It is a metalloid and one of the pnictogens, and therefore shares many properties with its group 15 neighbors phosphorus and antimony. Arsenic is a notoriously toxic heavy metal. It occurs naturally in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. It has various allotropes, but only the grey form, which has a metallic appearance, is important to industry.

Mineral wool is any fibrous material formed by spinning or drawing molten mineral or rock materials such as slag and ceramics.

Calcium sulfate (or calcium sulphate) is the inorganic compound with the formula CaSO4 and related hydrates. In the form of γ-anhydrite (the anhydrous form), it is used as a desiccant. One particular hydrate is better known as plaster of Paris, and another occurs naturally as the mineral gypsum. It has many uses in industry. All forms are white solids that are poorly soluble in water. Calcium sulfate causes permanent hardness in water.

Sawdust is a by-product or waste product of woodworking operations such as sawing, sanding, milling and routing. It is composed of very small chips of wood. These operations can be performed by woodworking machinery, portable power tools or by use of hand tools. In some manufacturing industries it can be a significant fire hazard and source of occupational dust exposure.

Antimony(III) oxide is the inorganic compound with the formula Sb2O3. It is the most important commercial compound of antimony. It is found in nature as the minerals valentinite and senarmontite. Like most polymeric oxides, Sb2O3 dissolves in aqueous solutions with hydrolysis. A mixed arsenic-antimony oxide occurs in nature as the very rare mineral stibioclaudetite.
Lead hydrogen arsenate, also called lead arsenate, acid lead arsenate or LA, chemical formula PbHAsO4, is an inorganic insecticide used primarily against the potato beetle. Lead arsenate was the most extensively used arsenical insecticide. Two principal formulations of lead arsenate were marketed: basic lead arsenate (Pb5OH(AsO4)3, CASN: 1327-31-7) and acid lead arsenate (PbHAsO4).
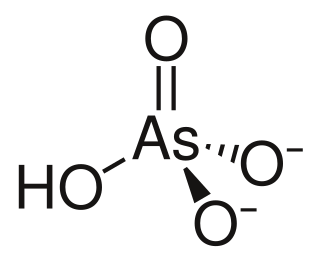
The arsenate is an ion with the chemical formula AsO3−4. Bonding in arsenate consists of a central arsenic atom, with oxidation state +5, double bonded to one oxygen atom and single bonded to a further three oxygen atoms. The four oxygen atoms orient around the arsenic atom in a tetrahedral geometry. Resonance disperses the ion's −3 charge across all four oxygen atoms.

Arsenic acid or arsoric acid is the chemical compound with the formula H3AsO4. More descriptively written as AsO(OH)3, this colorless acid is the arsenic analogue of phosphoric acid. Arsenate and phosphate salts behave very similarly. Arsenic acid as such has not been isolated, but is only found in solution, where it is largely ionized. Its hemihydrate form (2H3AsO4·H2O) does form stable crystals. Crystalline samples dehydrate with condensation at 100 °C.

Arsenous acid (or arsenious acid) is the inorganic compound with the formula H3AsO3. It is known to occur in aqueous solutions, but it has not been isolated as a pure material, although this fact does not detract from the significance of As(OH)3.

Picloram is a systemic herbicide used for general woody plant control. It also controls a wide range of broad-leaved weeds, but most grasses are resistant. A chlorinated derivative of picolinic acid, picloram is in the pyridine family of herbicides.
Arsenic pentoxide is the inorganic compound with the formula As2O5. This glassy, white, deliquescent solid is relatively unstable, consistent with the rarity of the As(V) oxidation state. More common, and far more important commercially, is arsenic(III) oxide (As2O3). All inorganic arsenic compounds are highly toxic and thus find only limited commercial applications.

Copper arsenate (Cu3(AsO4)2·4H2O, or Cu5H2(AsO4)4·2H2O), also called copper orthoarsenate, tricopper arsenate, cupric arsenate, or tricopper orthoarsenate, is a blue or bluish-green powder insoluble in water and alcohol and soluble in aqueous ammonium and dilute acids. Its CAS number is 7778-41-8 or 10103-61-4.
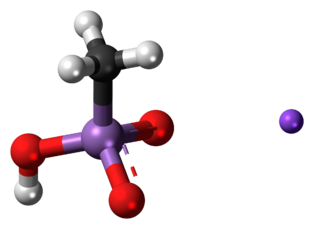
Monosodium methyl arsenate (MSMA) is an arsenic-based herbicide. It is an organo-arsenate; less toxic than the inorganic form of arsenates. However, the EPA states that all forms of arsenic are a serious risk to human health and the United States' Agency for Toxic Substances and Disease Registry ranked arsenic as number 1 in its 2001 Priority List of Hazardous Substances at Superfund sites.
Potassium arsenite (KAsO2) is an inorganic compound that exists in two forms, potassium meta-arsenite (KAsO2) and potassium ortho-arsenite (K3AsO3). It is composed of arsenite ions (AsO33− or AsO2−) with arsenic always existing in the +3 oxidation state. Like many other arsenic containing compounds, potassium arsenite is highly toxic and carcinogenic to humans. Potassium arsenite forms the basis of Fowler’s solution, which was historically used as a medicinal tonic, but due to its toxic nature its use was discontinued. Potassium arsenite is still, however, used as a rodenticide.

Calcium chromate is an inorganic compound with the formula CaCrO4, i.e. the chromate salt of calcium. It is a bright yellow solid which is normally found in the dihydrate form CaCrO4·2H2O. A very rare anhydrous mineral form exists in nature, which is known as chromatite.

Bromacil is an organic compound with the chemical formula C9H13BrN2O2, commercially available as a herbicide. Bromacil was first registered as a pesticide in the U.S. in 1961. It is used for brush control on non-cropland areas. It works by interfering with photosynthesis by entering the plant through the root zone and moving throughout the plant. Bromacil is one of a group of compounds called substituted uracils. These materials are broad spectrum herbicides used for nonselective weed and brush control on non-croplands, as well as for selective weed control on a limited number of crops, such as citrus fruit and pineapple. Bromacil is also found to be excellent at controlling perennial grasses.

Picropharmacolite, Ca4Mg(AsO3OH)2(AsO4)2·11H2O, is a rare arsenate mineral. It was named in 1819 from the Greek for bitter, in allusion to its magnesium content, and its chemical similarity to pharmacolite. The mineral irhtemite, Ca4Mg(AsO3OH)2(AsO4)2·4H2O, has the same composition as picropharmacolite, except that it has only four water molecules per formula unit, instead of eleven. It may be formed by the dehydration of picropharmacolite.

Vladimirite is a rare calcium arsenate mineral with a formula of Ca5(HAsO4)2(AsO4)2·5H2O. It is named after the Vladimirovskoye deposit in Russia, where it was discovered in the 1950s.
Ammonium orthomolybdate is the inorganic compound with the chemical formula (NH4)2MoO4. It is a white solid that is prepared by treating molybdenum trioxide with aqueous ammonia. Upon heating these solutions, ammonia is lost, to give ammonium heptamolybdate ((NH4)6Mo7O24·4H2O).

The arsenic (As) cycle is the biogeochemical cycle of natural and anthropogenic exchanges of arsenic terms through the atmosphere, lithosphere, pedosphere, hydrosphere, and biosphere. Although arsenic is naturally abundant in the Earth's crust, long-term exposure and high concentrations of arsenic can be detrimental to human health.



















