 | |
 | |
| Names | |
|---|---|
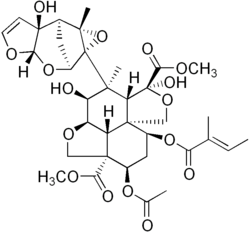
| Preferred IUPAC name Dimethyl (2aR,2a1R,3S,4S,4aR,5S,7aS,8S,10R,10aS)-10-(acetyloxy)-3,5-dihydroxy-4-[(1aR,2S,3aS,6aS,7S,7aS)-6a-hydroxy-7a-methyl-3a,6a,7,7a-tetrahydro-2,7-methanofuro[2,3-b]oxireno[2,3-e]oxepin-1a(2H)-yl]-4-methyl-8-{[(2E)-2-methylbut-2-enoyl]oxy}octahydro-1H,7H-naphtho[1,8-bc:4,4a-c′]difuran-5,10a(8H)-dicarboxylate | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.115.924 |
| KEGG | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C35H44O16 | |
| Molar mass | 720.721 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Azadirachtin, a chemical compound belonging to the limonoid group, is a secondary metabolite present in neem seeds. It is an insecticide used particularly in organic farming.
