A steroid is a biologically active organic compound with four fused rings arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes that alter membrane fluidity; and as signaling molecules. Hundreds of steroids are found in plants, animals and fungi. All steroids are manufactured in cells from the sterols lanosterol (opisthokonts) or cycloartenol (plants). Lanosterol and cycloartenol are derived from the cyclization of the triterpene squalene.

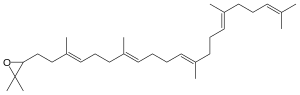
Squalene is an organic compound. It is a triterpenoid with the formula C30H50. It is a colourless oil, although impure samples appear yellow. It was originally obtained from shark liver oil (hence its name, as Squalus is a genus of sharks). An estimated 12% of bodily squalene in humans is found in sebum. Squalene has a role in topical skin lubrication and protection.

Hopanoids are a diverse subclass of triterpenoids with the same hydrocarbon skeleton as the compound hopane. This group of pentacyclic molecules therefore refers to simple hopenes, hopanols and hopanes, but also to extensively functionalized derivatives such as bacteriohopanepolyols (BHPs) and hopanoids covalently attached to lipid A.
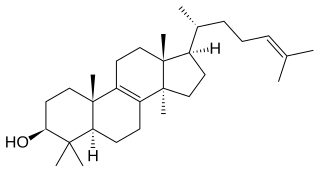
Lanosterol is a tetracyclic triterpenoid and is the compound from which all animal and fungal steroids are derived. By contrast plant steroids are produced via cycloartenol.

Lanosterol synthase (EC 5.4.99.7) is an oxidosqualene cyclase (OSC) enzyme that converts (S)-2,3-oxidosqualene to a protosterol cation and finally to lanosterol. Lanosterol is a key four-ringed intermediate in cholesterol biosynthesis. In humans, lanosterol synthase is encoded by the LSS gene.

Prenyltransferases (PTs) are a class of enzymes that transfer allylic prenyl groups to acceptor molecules. Prenyl transferases commonly refer to isoprenyl diphosphate syntheses (IPPSs). Prenyltransferases are a functional category and include several enzyme groups that are evolutionarily independent.
In enzymology, a cycloartenol synthase is an enzyme that catalyzes the chemical reaction

Integral monotopic proteins are permanently attached to the cell membrane from one side, and are a type of integral membrane protein (IMP).
Dammarenediol II synthase (EC 4.2.1.125, dammarenediol synthase, 2,3-oxidosqualene (20S)-dammarenediol cyclase, DDS, (S)-squalene-2,3-epoxide hydro-lyase (dammarenediol-II forming)) is an enzyme with systematic name (3S)-2,3-epoxy-2,3-dihydrosqualene hydro-lyase (dammarenediol-II forming). This enzyme catalyses the following chemical reaction
Squalene—hopanol cyclase (EC 4.2.1.129, squalene—hopene cyclase) is an enzyme with systematic name hopan-22-ol hydro-lyase. This enzyme catalyses the following chemical reaction

Squalene-hopene cyclase (SHC) (EC 5.4.99.17) or hopan-22-ol hydro-lyase is an enzyme in the terpene cyclase/mutase family. It catalyzes the interconversion of squalene into a pentacyclic triterpenes, hopene and hopanol. This enzyme catalyses the following chemical reactions.
β-amyrin synthase is an enzyme with systematic name (3S)-2,3-epoxy-2,3-dihydrosqualene mutase . This enzyme catalyses the following chemical reaction
Lupeol synthase is an enzyme with systematic name (3S)-2,3-epoxy-2,3-dihydrosqualene mutase . This enzyme catalyses the following chemical reaction
Shionone synthase is an enzyme with systematic name (3S)-2,3-epoxy-2,3-dihydrosqualene mutase . This enzyme catalyses the following chemical reaction
α-seco-amyrin synthase is an enzyme with systematic name (3S)-2,3-epoxy-2,3-dihydrosqualene mutase . This enzyme catalyses the following chemical reaction
β-seco-amyrin synthase is an enzyme with systematic name (3S)-2,3-epoxy-2,3-dihydrosqualene mutase . This enzyme catalyses the following chemical reaction
Baruol synthase is an enzyme with systematic name (3S)-2,3-epoxy-2,3-dihydrosqualene mutase . This enzyme catalyses the following chemical reaction

Parkeol is a relatively uncommon sterol secondary metabolite found mostly in plants, particularly noted in Butyrospermum parkii. It can be synthesized as a minor product by several oxidosqualene cyclase enzymes, and is the sole product of the enzyme parkeol synthase.

Oxidosqualene cyclases (OSC) are enzymes involved in cyclization reactions of 2,3-oxidosqualene to form sterols or triterpenes.

Taraxasterol (anthesterin) is a triterpene derived from the mevalonate pathway and is found in dandelions.