This article has multiple issues. Please help improve it or discuss these issues on the talk page . (Learn how and when to remove these messages)
|
 | |
 | |
 | |
| Names | |
|---|---|
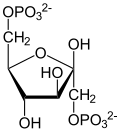
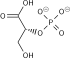
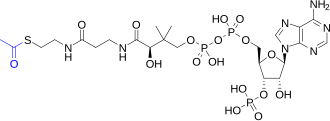
| Preferred IUPAC name O1-{(3R)-4-[(3-{[2-(Acetylsulfanyl)ethyl]amino}-3-oxopropyl)amino]-3-hydroxy-2,2-dimethyl-4-oxobutyl} O3-{[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methyl} dihydrogen diphosphate | |
| Identifiers | |
| |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.000.719 |
| KEGG | |
| MeSH | Acetyl+Coenzyme+A |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
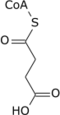
| C23H38N7O17P3S | |
| Molar mass | 809.57 g·mol−1 |
| UV-vis (λmax) | 260 nm; 232 nm [1] |
| Absorbance | ε260 = 16.4 mM−1 cm−1 (adenosine) [1] ε232 = 8.7 mM−1 cm−1 (thioester) [1] Δε232 on thioester hydrolysis = −4.5 mM−1 cm−1 [1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Acetyl-CoA (acetyl coenzyme A) is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. [2] Its main function is to deliver the acetyl group to the citric acid cycle (Krebs cycle) to be oxidized for energy production.
Contents
- Role
- Biosynthesis
- Extramitochondrial
- Intramitochondrial
- Functions
- Intermediates in various pathways
- See also
- References
- External links
Coenzyme A (CoASH or CoA) consists of a β-mercaptoethylamine group linked to pantothenic acid (vitamin B5) through an amide linkage [3] and 3'-phosphorylated ADP. The acetyl group (indicated in blue in the structural diagram on the right) of acetyl-CoA is linked to the sulfhydryl substituent of the β-mercaptoethylamine group. This thioester linkage is a "high energy" bond, which is particularly reactive. Hydrolysis of the thioester bond is exergonic (−31.5 kJ/mol).
CoA is acetylated to acetyl-CoA by the breakdown of carbohydrates through glycolysis and by the breakdown of fatty acids through β-oxidation. Acetyl-CoA then enters the citric acid cycle, where the acetyl group is oxidized to carbon dioxide and water, and the energy released is captured in the form of 11 ATP and one GTP per acetyl group.
Konrad Bloch and Feodor Lynen were awarded the 1964 Nobel Prize in Physiology or Medicine for their discoveries linking acetyl-CoA and fatty acid metabolism. Fritz Lipmann won the Nobel Prize in 1953 for his discovery of the cofactor coenzyme A. [4]