| |
 | |
| Clinical data | |
|---|---|
| Other names | 18-MC; Zolunicant; MM-110; MM110 |
| Routes of administration | Oral |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII |
|
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C22H28N2O3 |
| Molar mass | 368.477 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
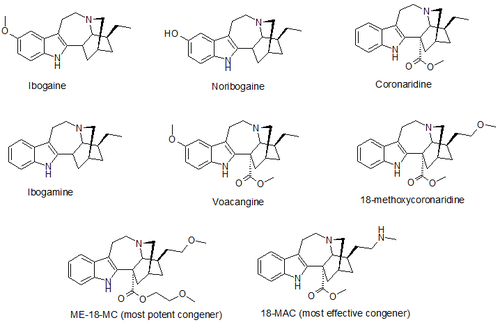
18-Methoxycoronaridine (18-MC; developmental code name MM-110), also known as zolunicant (INN ), is a derivative of ibogaine invented in 1996 by the research team around the pharmacologist Stanley D. Glick from the Albany Medical College and the chemists Upul K. Bandarage and Martin E. Kuehne from the University of Vermont. In animal studies it has proven to be effective at reducing self-administration of morphine, cocaine, methamphetamine, nicotine, and sucrose. [1] [2] It has also been shown to produce anorectic effects in obese rats, most likely due to the same actions on the reward system which underlie its anti-addictive effects against drug addiction. [3]
Contents
18-MC was originally developed by Savant HWP and later acquired by MindMed in 2019 for development as a treatment for opioid use disorder. [4] A Phase 1 trial in healthy volunteers was completed in 2022 with favorable safety and tolerability. [5] Due to strategic reprioritization, MindMed discontinued active development of MM-110 in 2023 and has been seeking non-dilutive funding or partners to potentially restart the program; as of 2025 the program remains shelved. [6] [7] A separate Phase 2 trial in Brazil for cutaneous leishmaniasis (initiated 2017) has unknown status with no published results. [8]