
Dextromethorphan, or DXM, a common active ingredient found in many over-the-counter cough suppressant cold medicines, is used as a recreational drug and entheogen for its dissociative effects. It has almost no psychoactive effects at medically recommended doses. However, dextromethorphan has powerful dissociative properties when administered in doses well above those considered therapeutic for cough suppression. Recreational use of DXM is sometimes referred to in slang form as "robo-tripping", whose prefix derives from the Robitussin brand name, or "Triple Cs", which derives from the Coricidin brand whose tablets are printed with "CC+C" for "Coricidin Cough and Cold". However, this brand presents additional danger when used at recreational doses due to the presence of chlorpheniramine.

Ipratropium bromide, sold under the trade name Atrovent among others, is a type of anticholinergic medication which opens up the medium and large airways in the lungs. It is used to treat the symptoms of chronic obstructive pulmonary disease and asthma. It is used by inhaler or nebulizer. Onset of action is typically within 15 to 30 minutes and lasts for three to five hours.

Guaifenesin, also known as glyceryl guaiacolate, is an expectorant medication taken by mouth and marketed as an aid to eliminate sputum from the respiratory tract. Chemically, it is an ether of guaiacol and glycerine. It may be used in combination with other medications. A 2014 study found that guaifenesin has no effect on sputum production or clearance in upper respiratory infections.

Promethazine, sold under the brand name Phenergan among others, is a first-generation antihistamine, antipsychotic, sedative, and antiemetic used to treat allergies, insomnia, and nausea. It may also help with some symptoms associated with the common cold and may also be used for sedating people who are agitated or anxious, an effect that has led to some recreational use. Promethazine is taken by mouth (oral), as a rectal suppository, or by injection into a muscle (IM).

Dihydrocodeine is a semi-synthetic opioid analgesic prescribed for pain or severe dyspnea, or as an antitussive, either alone or compounded with paracetamol (acetaminophen) or aspirin. It was developed in Germany in 1908 and first marketed in 1911.

Dextrorphan (DXO) is a psychoactive drug of the morphinan class which acts as an antitussive or cough suppressant and dissociative hallucinogen. It is the dextrorotatory enantiomer of racemorphan; the levorotatory enantiomer is levorphanol. Dextrorphan is produced by O-demethylation of dextromethorphan by CYP2D6. Dextrorphan is an NMDA antagonist and contributes to the psychoactive effects of dextromethorphan.

Phenyltoloxamine is an antihistamine with sedative and analgesic effects. It is available in combination with other drugs such as paracetamol (acetominophen).

Dextromethorphan (DXM) is a cough suppressant used in many cough and cold medicines. It affects NMDA, and sigma-1 receptors in the brain, all of which have been implicated in the pathophysiology of depression. In 2022, the FDA approved a formulation of it combined with bupropion named Auvelity to serve as a rapid acting antidepressant in patients with major depressive disorder.

Levorphanol is an opioid medication used to treat moderate to severe pain. It is the levorotatory enantiomer of the compound racemorphan. Its dextrorotatory counterpart is dextrorphan.
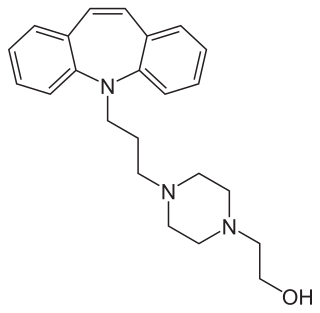
Opipramol, sold under the brand name Insidon among others, is an anxiolytic and tricyclic antidepressant that is used throughout Europe. Despite chemically being a tricyclic dibenzazepine (iminostilbene) derivative similar to imipramine, opipramol is not a monoamine reuptake inhibitor like most other tricyclic antidepressants, and instead, uniquely among antidepressants, acts primarily as a SIGMAR1 agonist. It was developed by Schindler and Blattner in 1961.

Thebacon, or dihydrocodeinone enol acetate, is a semisynthetic opioid that is similar to hydrocodone and is most commonly synthesised from thebaine. Thebacon was invented in Germany in 1924, four years after the first synthesis of hydrocodone. Thebacon is a derivative of acetyldihydrocodeine, where only the 6–7 double bond is saturated. Thebacon is marketed as its hydrochloride salt under the trade name Acedicon, and as its bitartrate under Diacodin and other trade names. The hydrochloride salt has a free base conversion ratio of 0.846. Other salts used in research and other settings include thebacon's phosphate, hydrobromide, citrate, hydroiodide, and sulfate.
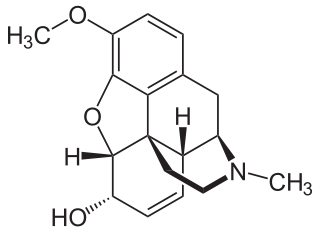
Codeine is an opiate and prodrug of morphine mainly used to treat pain, coughing, and diarrhea. It is also commonly used as a recreational drug. It is found naturally in the sap of the opium poppy, Papaver somniferum. It is typically used to treat mild to moderate degrees of pain. Greater benefit may occur when combined with paracetamol (acetaminophen) or a nonsteroidal anti-inflammatory drug (NSAID) such as aspirin or ibuprofen. Evidence does not support its use for acute cough suppression in children or adults. In Europe, it is not recommended as a cough medicine in those under 12 years of age. It is generally taken by mouth. It typically starts working after half an hour, with maximum effect at two hours. Its effects last for about four to six hours. Codeine exhibits abuse potential similar to other opioid medications, including a risk of habituation and overdose.
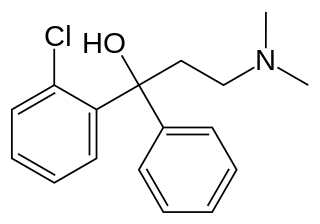
Clofedanol (INN) or chlophedianol (BAN), sold under the brand name Ninjacof among others, is a centrally acting cough suppressant used in the treatment of dry cough. Clofedanol has local anesthetic, antispasmodic, and antihistamine properties, and may have anticholinergic effects at high doses.

Cloperastine (INN) or cloperastin, in the forms of cloperastine hydrochloride (JAN) and cloperastine fendizoate, is an antitussive and antihistamine that is marketed as a cough suppressant in Japan, Hong Kong, and in some European countries. It was first introduced in 1972 in Japan, and then in Italy in 1981.

Zipeprol is a centrally acting cough suppressant developed in France in the 1970s. It is not a morphinan derivative. Zipeprol acts as a local anaesthetic and has mucolytic, antihistamine and anticholinergic properties. It is sold with several brand names such as Zinolta and Respilene. It is not available in the United States or Canada and has been discontinued in Europe. It is still available in some countries in Asia and South America.

Butamirate is a cough suppressant. It has been marketed in Europe and Mexico, but not in the United States.

Dimemorfan (INN), or dimemorfan phosphate (JAN), also known as 3,17-dimethylmorphinan, is an antitussive of the morphinan family that is widely used in Japan and is also marketed in Spain and Italy. It was developed by Yamanouchi Pharmaceutical and introduced in Japan in 1975. It was later introduced in Spain in 1981 and Japan in 1985.

Racemorphan, or morphanol, is the racemic mixture of the two stereoisomers of 17-methylmorphinan-3-ol, each with differing pharmacology and effects:

Caramiphen is an anticholinergic drug used in the treatment of Parkinson's disease. In combination with phenylpropanolamine it is used as a cough suppressant and nasal decongestant to treat symptoms associated with respiratory illnesses such as cold, allergies, hay fever, and sinusitis. It was added to the British National Formulary in 1963, with a dosage of 10 to 20 mg. Side effects include nausea, dizziness, and drowsiness.

Fominoben is an antitussive agent of the benzanilide class, formerly marketed under the name Noleptan. It binds poorly to the sigma-1 receptor, a receptor activated by many other antitussives. It is reported to have respiratory stimulant activity. Other research has indicated it may be an agonist at the benzodiazepine site of the GABAA receptor. It was introduced in Germany in 1973, in Italy in 1979, and in Japan in 1983.




















