Medicines for pain and palliative care
Non-opioids and non-steroidal anti-inflammatory medicines (NSAIMs)

- Acetylsalicylic acid (aspirin)
- Ibuprofen [note 5]
- Paracetamol (acetaminophen) [note 6]
Opioid analgesics
Complementary:
The WHO Model List of Essential Medicines (a.k.a. Essential Medicines List or EML [1] ), published by the World Health Organization (WHO), contains the medications considered to be most effective and safe to meet the most important needs in a health system. [2] The list is frequently used by countries to help develop their own local lists of essential medicines. [2] As of 2016 [update] , more than 155 countries have created national lists of essential medicines based on the World Health Organization's model list. [1] This includes both developed and developing countries. [2] [3]
The list is divided into core items and complementary items. [4] The core items are deemed to be the most cost-effective options for key health problems and are usable with little additional health care resources. [4] The complementary items either require additional infrastructure such as specially trained health care providers or diagnostic equipment or have a lower cost–benefit ratio. [4] About 25% of items are in the complementary list. [5] Some medications are listed as both core and complementary. [6] While most medications on the list are available as generic products, being under patent does not prevent inclusion. [7]
The first list was published in 1977 and included 208 medications. [8] [2] [9] The WHO updates the list every two years. [10] There are 306 medications in the 14th list in 2005, [11] 410 in the 19th list in 2015, [10] 433 in the 20th list in 2017, [12] [13] 460 in the 21st list in 2019, [14] [15] [16] and 479 in the 22nd list in 2021. [17] [18] Various national lists contain between 334 and 580 medications. [5] [19] The Essential Medicines List (EML) was updated in September 2025 to its 24th edition. [20] The list contains recommendations for 523 medications.
A separate list for children up to twelve years of age, known as the WHO Model List of Essential Medicines for Children (EMLc), was created in 2007, and is in its 10th edition. [10] [21] [22] [23] [24] It was created to make sure that the needs of children were systematically considered such as availability of proper formulations. [25] [26] Everything in the children's list is also included in the main list. [27] The list and notes are based on the 19th to 24th edition of the main list. [4] [12] [14] [17] [28] Therapeutic alternatives with similar clinical performance are listed for some medicines and they may be considered for national essential medicines lists. [17] [18] The 10th Essential Medicines List for Children was updated in September 2025. [24] [29] [30]
Note: An α indicates a medicine is on the complementary list. [4] [14] [17]
Complementary:

Complementary:
Complementary:
Complementary:
Complementary:
Complementary:
Complementary:
Complementary:
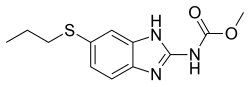
Complementary:
Complementary:
This group includes antibiotics that have activity against a wide range of commonly encountered susceptible pathogens while also showing lower resistance potential than antibiotics in the other groups. [20]
This group includes antibiotic classes that have higher resistance potential and includes most of the highest priority agents among the Critically Important Antimicrobials for Human Medicine and/or antibiotics that are at relatively high risk of selection of bacterial resistance. [20]
Complementary:
This group includes antibiotics and antibiotic classes that should be reserved for treatment of confirmed or suspected infections due to multi-drug-resistant organisms. [20] Complementary:

Complementary:
Complementary:
Complementary:
Complementary:
No listings in this section.
Complementary:
Complementary:
Complementary:
Complementary:
Complementary:
Complementary:
Complementary:
Complementary:
Complementary:
Complementary:
Complementary:
Complementary:
Complementary:
Complementary:
Complementary:
Complementary:
Complementary:
Complementary:
Complementary:
Complementary:
Complementary:
Complementary:
Complementary:
No listings in this section.
Complementary:
Complementary:
Complementary:
Complementary:
Complementary:
Complementary:
Complementary:
Complementary:
Complementary:
Complementary:
Complementary:
Complementary:
Complementary:
Complementary:
Complementary:
An α indicates the medicine is on the complementary list for which specialized diagnostic or monitoring or training is needed. An item may also be listed as complementary on the basis of higher costs or a less attractive cost-benefit ratio. [4] [14]
The current versions are the 21st WHO Essential Medicines List (EML) and the 7th WHO Essential Medicines List for Children (EMLc) updated in June 2019.
{{cite book}}: CS1 maint: location missing publisher (link){{cite book}}: CS1 maint: location missing publisher (link){{cite book}}: CS1 maint: location missing publisher (link){{cite book}}: CS1 maint: location missing publisher (link)