
Leukemia is a group of blood cancers that usually begin in the bone marrow and produce high numbers of abnormal blood cells. These blood cells are not fully developed and are called blasts or leukemia cells. Symptoms may include bleeding and bruising, bone pain, fatigue, fever, and an increased risk of infections. These symptoms occur due to a lack of normal blood cells. Diagnosis is typically made by blood tests or bone marrow biopsy.

A myelodysplastic syndrome (MDS) is one of a group of cancers in which blood cells in the bone marrow do not mature, and as a result, do not develop into healthy blood cells. Early on, no symptoms typically are seen. Later, symptoms may include fatigue, shortness of breath, bleeding disorders, anemia, or frequent infections. Some types may develop into acute myeloid leukemia.

Chronic lymphocytic leukemia (CLL) is a type of cancer that affects the blood and bone marrow. In CLL, the bone marrow makes too many lymphocytes, which are a type of white blood cell. Many people do not have any symptoms when they are first diagnosed. Those with symptoms may experience fevers, fatigue, night sweats, and weight loss. These patients may also have painless lymph node swelling, enlargement of the spleen, and/or a low red blood cell count (anemia). These symptoms may worsen over time.
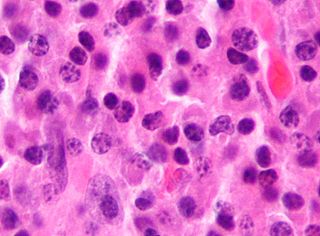
Tumors of the hematopoietic and lymphoid tissues or tumours of the haematopoietic and lymphoid tissues are tumors that affect the blood, bone marrow, lymph, and lymphatic system. Because these tissues are all intimately connected through both the circulatory system and the immune system, a disease affecting one will often affect the others as well, making aplasia, myeloproliferation and lymphoproliferation closely related and often overlapping problems. While uncommon in solid tumors, chromosomal translocations are a common cause of these diseases. This commonly leads to a different approach in diagnosis and treatment of hematological malignancies. Hematological malignancies are malignant neoplasms ("cancer"), and they are generally treated by specialists in hematology and/or oncology. In some centers "hematology/oncology" is a single subspecialty of internal medicine while in others they are considered separate divisions. Not all hematological disorders are malignant ("cancerous"); these other blood conditions may also be managed by a hematologist.

Cytarabine, also known as cytosine arabinoside (ara-C), is a chemotherapy medication used to treat acute myeloid leukemia (AML), acute lymphocytic leukemia (ALL), chronic myelogenous leukemia (CML), and non-Hodgkin's lymphoma. It is given by injection into a vein, under the skin, or into the cerebrospinal fluid. There is a liposomal formulation for which there is tentative evidence of better outcomes in lymphoma involving the meninges.

Rasburicase, sold under the brand name Elitek in the US and Fasturtec in the EU, is a medication that helps to clear uric acid from the blood. It is a recombinant version of urate oxidase, an enzyme that metabolizes uric acid to allantoin. Urate oxidase is known to be present in many mammals but does not naturally occur in humans. Rasburicase is produced by a genetically modified Saccharomyces cerevisiae strain. The complementary DNA (cDNA) coding for rasburicase was cloned from a strain of Aspergillus flavus.

Rituximab, sold under the brand name Rituxan among others, is a monoclonal antibody medication used to treat certain autoimmune diseases and types of cancer. It is used for non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis, granulomatosis with polyangiitis, idiopathic thrombocytopenic purpura, pemphigus vulgaris, myasthenia gravis and Epstein–Barr virus-positive mucocutaneous ulcers. It is given by slow intravenous infusion.

Alemtuzumab, sold under the brand names Campath and Lemtrada among others, is a medication used to treat chronic lymphocytic leukemia and multiple sclerosis. In chronic lymphocytic leukemia, it has been used as both a first line and second line treatment. It is given by injection into a vein.

Chlorambucil, sold under the brand name Leukeran among others, is a chemotherapy medication used to treat chronic lymphocytic leukemia (CLL), Hodgkin lymphoma, and non-Hodgkin lymphoma. For CLL it is a preferred treatment. It is given by mouth.
Autoimmune hemolytic anemia (AIHA) is an autoimmune disorder which occurs when antibodies directed against the person's own red blood cells (RBCs) cause them to burst (lyse), leading to an insufficient number of oxygen-carrying red blood cells in circulation (anemia). The lifetime of the RBCs is reduced from the normal 100–120 days to just a few days in serious cases. The intracellular components of the RBCs are released into the circulating blood and into tissues, leading to some of the characteristic symptoms of this condition. The antibodies are usually directed against high-incidence antigens, therefore they also commonly act on allogenic RBCs. AIHA is a relatively rare condition, with an incidence of 5–10 cases per 1 million persons per year in the warm-antibody type and 0.45 to 1.9 cases per 1 million persons per year in the cold-antibody type. Autoimmune hemolysis might be a precursor of later onset systemic lupus erythematosus.
Paroxysmal cold hemoglobinuria (PCH) or Donath–Landsteiner hemolytic anemia (DLHA) is an autoimmune hemolytic anemia featured by complement-mediated intravascular hemolysis after cold exposure. It can present as an acute non-recurrent postinfectious event in children, or chronic relapsing episodes in adults with hematological malignancies or tertiary syphilis. Described by Julius Donath (1870–1950) and Karl Landsteiner (1868–1943) in 1904, PCH is one of the first clinical entities recognized as an autoimmune disorder.

Bendamustine, sold under the brand name Treanda among others, is a chemotherapy medication used in the treatment of chronic lymphocytic leukemia (CLL), multiple myeloma, and non-Hodgkin's lymphoma. It is given by injection into a vein.
Hematologic diseases are disorders which primarily affect the blood and blood-forming organs. Hematologic diseases include rare genetic disorders, anemia, HIV, sickle cell disease and complications from chemotherapy or transfusions.
Obinutuzumab, sold under the brand name Gazyva among others, is a humanized anti-CD20 monoclonal antibody used as a treatment for cancer. It was originated by GlycArt Biotechnology AG and developed by Roche.
Acquired hemolytic anemia can be divided into immune and non-immune mediated forms of hemolytic anemia.
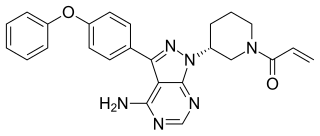
Ibrutinib, sold under the brand name Imbruvica among others, is a small molecule drug that inhibits B-cell proliferation and survival by irreversibly binding the protein Bruton's tyrosine kinase (BTK). Blocking BTK inhibits the B-cell receptor pathway, which is often aberrantly active in B cell cancers. Ibrutinib is therefore used to treat such cancers, including mantle cell lymphoma, chronic lymphocytic leukemia, and Waldenström's macroglobulinemia. Ibrutinib also binds to C-terminal Src Kinases. These are off-target receptors for the BTK inhibitor. Ibrutinib binds to these receptors and inhibits the kinase from promoting cell differentiation and growth. This leads to many different side effects like left atrial enlargement and atrial fibrillation during the treatment of Chronic Lymphocytic Leukemia.
FCM, or FMC in the context of chemotherapy is an acronym for a chemotherapy regimen that is used in the treatment of indolent B cell non-Hodgkin's lymphomas. In combination with Rituximab, this regimen is called R-FCM or R-FMC, or FCM-R, FMC-R.
FLAG is a chemotherapy regimen used for relapsed and refractory acute myeloid leukemia (AML). The acronym incorporates the three primary ingredients of the regimen:
- Fludarabine: an antimetabolite that, while not active toward AML, increases formation of an active cytarabine metabolite, ara-CTP, in AML cells;
- Arabinofuranosyl cytidine : an antimetabolite that has been proven to be the most active toward AML among various cytotoxic drugs in single-drug trials; and
- Granulocyte colony-stimulating factor (G-CSF): a glycoprotein that shortens the duration and severity of neutropenia.

Venetoclax, sold under the brand names Venclexta and Venclyxto, is a medication used to treat adults with chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL), or acute myeloid leukemia (AML).












