 | |
| Clinical data | |
|---|---|
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 96 to 98% |
| Elimination half-life | 8–9 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.051.887 |
| Chemical and physical data | |
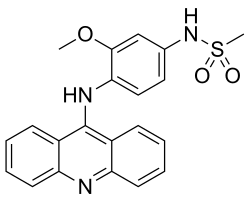
| Formula | C21H19N3O3S |
| Molar mass | 393.46 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Amsacrine (synonyms: m-AMSA, acridinyl anisidide) is an antineoplastic agent.
It has been used in acute lymphoblastic leukemia. [1]