Peptidoglycan or murein is a unique large macromolecule, a polysaccharide, consisting of sugars and amino acids that forms a mesh-like peptidoglycan layer (sacculus) that surrounds the bacterial cytoplasmic membrane. The sugar component consists of alternating residues of β-(1,4) linked N-acetylglucosamine (NAG) and N-acetylmuramic acid (NAM). Attached to the N-acetylmuramic acid is an oligopeptide chain made of three to five amino acids. The peptide chain can be cross-linked to the peptide chain of another strand forming the 3D mesh-like layer. Peptidoglycan serves a structural role in the bacterial cell wall, giving structural strength, as well as counteracting the osmotic pressure of the cytoplasm. This repetitive linking results in a dense peptidoglycan layer which is critical for maintaining cell form and withstanding high osmotic pressures, and it is regularly replaced by peptidoglycan production. Peptidoglycan hydrolysis and synthesis are two processes that must occur in order for cells to grow and multiply, a technique carried out in three stages: clipping of current material, insertion of new material, and re-crosslinking of existing material to new material.
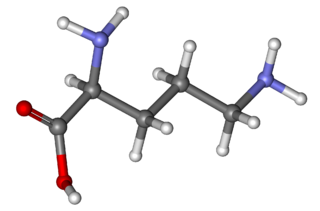
Ornithine is a non-proteinogenic α-amino acid that plays a role in the urea cycle. Ornithine is abnormally accumulated in the body in ornithine transcarbamylase deficiency. The radical is ornithyl.

In molecular biology and genetics, transformation is the genetic alteration of a cell resulting from the direct uptake and incorporation of exogenous genetic material from its surroundings through the cell membrane(s). For transformation to take place, the recipient bacterium must be in a state of competence, which might occur in nature as a time-limited response to environmental conditions such as starvation and cell density, and may also be induced in a laboratory.

The rifamycins are a group of antibiotics that are synthesized either naturally by the bacterium Amycolatopsis rifamycinica or artificially. They are a subclass of the larger family of ansamycins. Rifamycins are particularly effective against mycobacteria, and are therefore used to treat tuberculosis, leprosy, and mycobacterium avium complex (MAC) infections.

Legionella pneumophila is an aerobic, pleomorphic, flagellated, non-spore-forming, Gram-negative bacterium of the genus Legionella. L. pneumophila is the primary human pathogenic bacterium in this group. In nature, L. pneumophila infects freshwater and soil amoebae of the genera Acanthamoeba and Naegleria. This pathogen is found commonly near freshwater environments and will then invade the amoebae found in these environments, using them to carry out metabolic functions.

Clavulanic acid is a β-lactam drug that functions as a mechanism-based β-lactamase inhibitor. While not effective by itself as an antibiotic, when combined with penicillin-group antibiotics, it can overcome antibiotic resistance in bacteria that secrete β-lactamase, which otherwise inactivates most penicillins.
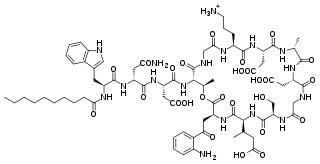
Daptomycin, sold under the brand name Cubicin among others, is a lipopeptide antibiotic used in the treatment of systemic and life-threatening infections caused by Gram-positive organisms.

Borrelia burgdorferi is a bacterial species of the spirochete class in the genus Borrelia, and is one of the causative agents of Lyme disease in humans. Along with a few similar genospecies, some of which also cause Lyme disease, it makes up the species complex of Borrelia burgdorferi sensu lato. The complex currently comprises 20 accepted and 3 proposed genospecies. B. burgdorferi sensu stricto exists in North America and Eurasia and until 2016 was the only known cause of Lyme disease in North America. Borrelia species are Gram-negative.
Shikimic acid, more commonly known as its anionic form shikimate, is a cyclohexene, a cyclitol and a cyclohexanecarboxylic acid. It is an important biochemical metabolite in plants and microorganisms. Its name comes from the Japanese flower shikimi, from which it was first isolated in 1885 by Johan Fredrik Eykman. The elucidation of its structure was made nearly 50 years later.
Production of antibiotics is a naturally occurring event, that thanks to advances in science can now be replicated and improved upon in laboratory settings. Due to the discovery of penicillin by Alexander Fleming, and the efforts of Florey and Chain in 1938, large-scale, pharmaceutical production of antibiotics has been made possible. As with the initial discovery of penicillin, most antibiotics have been discovered as a result of happenstance. Antibiotic production can be grouped into three methods: natural fermentation, semi-synthetic, and synthetic. As more and more bacteria continue to develop resistance to currently produced antibiotics, research and development of new antibiotics continues to be important. In addition to research and development into the production of new antibiotics, repackaging delivery systems is important to improving efficacy of the antibiotics that are currently produced. Improvements to this field have seen the ability to add antibiotics directly into implanted devices, aerosolization of antibiotics for direct delivery, and combination of antibiotics with non antibiotics to improve outcomes. The increase of antibiotic resistant strains of pathogenic bacteria has led to an increased urgency for the funding of research and development of antibiotics and a desire for production of new and better acting antibiotics.

Novobiocin, also known as albamycin or cathomycin, is an aminocoumarin antibiotic that is produced by the actinomycete Streptomyces niveus, which has recently been identified as a subjective synonym for S. spheroides a member of the class Actinomycetia. Other aminocoumarin antibiotics include clorobiocin and coumermycin A1. Novobiocin was first reported in the mid-1950s.

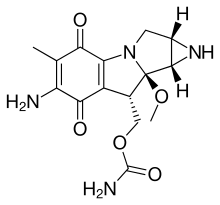
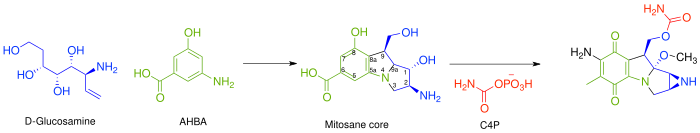
Mitomycin C is a mitomycin that is used as a chemotherapeutic agent by virtue of its antitumour activity.

In microbiology, genetics, cell biology, and molecular biology, competence is the ability of a cell to alter its genetics by taking up extracellular ("naked") DNA from its environment in the process called transformation. Competence may be differentiated between natural competence, a genetically specified ability of bacteria which is thought to occur under natural conditions as well as in the laboratory, and induced or artificial competence, which arises when cells in laboratory cultures are treated to make them transiently permeable to DNA. Competence allows for rapid adaptation and DNA repair of the cell. This article primarily deals with natural competence in bacteria, although information about artificial competence is also provided.

Geldanamycin is a 1,4-benzoquinone ansamycin antitumor antibiotic that inhibits the function of Hsp90 by binding to the unusual ADP/ATP-binding pocket of the protein. HSP90 client proteins play important roles in the regulation of the cell cycle, cell growth, cell survival, apoptosis, angiogenesis and oncogenesis.

Prodigiosin is the red dyestuff produced by many strains of the bacterium Serratia marcescens, as well as other Gram-negative, gamma proteobacteria such as Vibrio psychroerythrus and Hahella chejuensis. It is responsible for the pink tint occasionally found in grime that accumulates on porcelain surfaces such as bathtubs, sinks, and toilet bowls. It is in the prodiginine family of compounds which are produced in some Gram-negative gamma proteobacteria, as well as select Gram-positive Actinobacteria. The name prodigiosin is derived from prodigious.

Virulence-related outer membrane proteins, or outer surface proteins (Osp) in some contexts, are expressed in the outer membrane of gram-negative bacteria and are essential to bacterial survival within macrophages and for eukaryotic cell invasion.

Aminoshikimic acid is a synthetic crystalline carboxylic acid. It is characterized by multiple stereogenic centers and functional groups arrayed around a six-membered carbocyclic ring. Aminoshikimic acid is also an alternative to shikimic acid as a starting material for the synthesis of neuraminidase inhibitors such as the antiinfluenza agent oseltamivir (Tamiflu).
The origin and function of meiosis are currently not well understood scientifically, and would provide fundamental insight into the evolution of sexual reproduction in eukaryotes. There is no current consensus among biologists on the questions of how sex in eukaryotes arose in evolution, what basic function sexual reproduction serves, and why it is maintained, given the basic two-fold cost of sex. It is clear that it evolved over 1.2 billion years ago, and that almost all species which are descendants of the original sexually reproducing species are still sexual reproducers, including plants, fungi, and animals.
Naphthomycins are a group of closely related antimicrobial chemical compounds isolated from Streptomyces. They are considered a subclass of ansamycins.
Borrelia mayonii is a Gram-negative, host-associated spirochete that is capable of causing Lyme disease. This organism can infect various vertebrate hosts such as humans via the bite of a black legged tick.