
Liposarcomas are the most common subtype of soft tissue sarcomas, accounting for at least 20% of all sarcomas in adults. Soft tissue sarcomas are rare neoplasms with over 150 different histological subtypes or forms. Liposarcomas arise from the precursor lipoblasts of the adipocytes in adipose tissues. Adipose tissues are distributed throughout the body, including such sites as the deep and more superficial layers of subcutaneous tissues as well as in less surgically accessible sites like the retroperitoneum and visceral fat inside the abdominal cavity.
Bevacizumab, sold under the brand name Avastin among others, is a monoclonal antibody medication used to treat a number of types of cancers and a specific eye disease. For cancer, it is given by slow injection into a vein (intravenous) and used for colon cancer, lung cancer, ovarian cancer, glioblastoma, hepatocellular carcinoma, and renal-cell carcinoma. In many of these diseases it is used as a first-line therapy. For age-related macular degeneration it is given by injection into the eye (intravitreal).

Doxorubicin, sold under the brand name Adriamycin among others, is a chemotherapy medication used to treat cancer. This includes breast cancer, bladder cancer, Kaposi's sarcoma, lymphoma, and acute lymphocytic leukemia. It is often used together with other chemotherapy agents. Doxorubicin is given by injection into a vein.

Melphalan, sold under the brand name Alkeran among others, is a chemotherapy medication used to treat multiple myeloma; malignant lymphoma; lymphoblastic and myeloblastic leukemia; childhood neuroblastoma; ovarian cancer; mammary adenocarcinoma; and uveal melanoma. It is taken by mouth or by injection into a vein.

Dactinomycin, also known as actinomycin D, is a chemotherapy medication used to treat a number of types of cancer. This includes Wilms tumor, rhabdomyosarcoma, Ewing's sarcoma, trophoblastic neoplasm, testicular cancer, and certain types of ovarian cancer. It is given by injection into a vein.

Topotecan, sold under the brand name Hycamtin among others, is a chemotherapeutic agent medication that is a topoisomerase inhibitor. It is a synthetic, water-soluble analog of the natural chemical compound camptothecin. It is used in the form of its hydrochloride salt to treat ovarian cancer, lung cancer and other cancer types.

Ifosfamide (IFO), sold under the brand name Ifex among others, is a chemotherapy medication used to treat a number of types of cancer. This includes testicular cancer, soft tissue sarcoma, osteosarcoma, bladder cancer, small cell lung cancer, cervical cancer, and ovarian cancer. It is administered by injection into a vein.

Didemnins are cyclic depsipeptide compounds isolated from a tunicate of the genus Trididemnum that were collected in the Caribbean Sea. They were first isolated in 1978 at the University of Illinois.
Pazopanib, sold under the brand name Votrient, is an anti-cancer medication marketed worldwide by Novartis. It is a potent and selective multi-targeted receptor tyrosine kinase inhibitor that blocks tumour growth and inhibits angiogenesis. It has been approved for renal cell carcinoma and soft tissue sarcoma by numerous regulatory administrations worldwide.

Eribulin, sold under the brand name Halaven among others, is an anti-cancer medication used to treat breast cancer and liposarcoma.

PARP inhibitors are a group of pharmacological inhibitors of the enzyme poly ADP ribose polymerase (PARP).

Rucaparib, sold under the brand name Rubraca, is a PARP inhibitor used as an anti-cancer agent. Rucaparib is a first-in-class pharmaceutical drug targeting the DNA repair enzyme poly-ADP ribose polymerase-1 (PARP-1). It is taken by mouth.
Olaratumab, sold under the brand name Lartruvo, is a monoclonal antibody medication developed by Eli Lilly and Company for the treatment of solid tumors. It is directed against the platelet-derived growth factor receptor alpha.

Ecteinascidia turbinata, commonly known as the mangrove tunicate, is a species of tunicate in the family Perophoridae. It was described to science in 1880 by William Abbott Herdman. The cancer drug trabectedin can be isolated from this species.

Binimetinib, sold under the brand name Mektovi, is an anti-cancer medication used to treat various cancers. Binimetinib is a selective inhibitor of MEK, a central kinase in the tumor-promoting MAPK pathway. Inappropriate activation of the pathway has been shown to occur in many cancers. In June 2018 it was approved by the FDA in combination with encorafenib for the treatment of patients with unresectable or metastatic BRAF V600E or V600K mutation-positive melanoma. In October 2023, it was approved by the FDA for treatment of NSCLC with a BRAF V600E mutation in combination with encorafenib. It was developed by Array Biopharma.
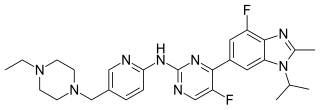
Abemaciclib, sold under the brand name Verzenio among others, is a medication for the treatment of advanced or metastatic breast cancers. It was developed by Eli Lilly and it acts as a CDK inhibitor selective for CDK4 and CDK6.

Mirvetuximab soravtansine, sold under the brand name Elahere, is a medication used as a treatment for epithelial ovarian cancer, fallopian tube cancer, or primary peritoneal cancer. Mirvetuximab soravtansine is a folate receptor alpha directed antibody and microtubule inhibitor conjugate.
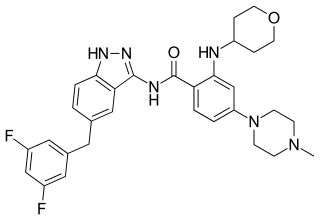
Entrectinib, sold under the brand name Rozlytrek, is an anti-cancer medication used to treat ROS1-positive non-small cell lung cancer and NTRK fusion-positive solid tumors. It is a selective tyrosine kinase inhibitor (TKI), of the tropomyosin receptor kinases (TRK) A, B and C, C-ros oncogene 1 (ROS1) and anaplastic lymphoma kinase (ALK).

PharmaMar is a Spanish pharmaceutical company headquartered in Colmenar Viejo, Madrid, Spain. Founded in 1986 as a subsidiary of Zeltia, it absorbed its parent company and all its subsidiaries in a reverse merger takeover in 2015. The company is a component of the Madrid Stock Exchange General Index (IGBM) and the IBEX 35 since 2020, after being part of the Ibex Small Cap stock market index.

Lurbinectedin, sold under the brand name Zepzelca, is a medication used for the treatment of small cell lung cancer.


















