Related Research Articles

The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of pharmaceutical products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or European Medicines Evaluation Agency (EMEA).
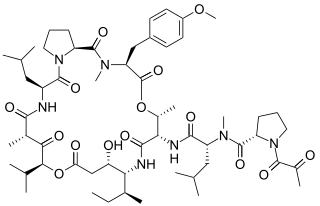
Plitidepsin is a chemical compound extracted from the ascidian Aplidium albicans. It is currently undergoing clinical trial testing. It is a member of the class of compounds known as didemnins.
Ustekinumab, sold under the brand name Stelara among others, is a monoclonal antibody medication developed by Janssen Pharmaceuticals, for the treatment of Crohn's disease, ulcerative colitis, plaque psoriasis and psoriatic arthritis, targeting both IL-12 and IL-23.
Paediatric-use marketing authorisations (PUMA) are granted by the European Medicines Agency (EMA) for medical products that are intended exclusively for paediatric use, that is, for use in patients younger than 18 years. Like ordinary EMA marketing authorisations, a PUMA approval is valid in all countries of the European Economic Area. The PUMA process was established to make it more profitable for pharmaceutical companies to market drugs for children. For this purpose, new data used for PUMA approved drugs are protected for 10 years, and the applications are partially exempt from fees.
Obiltoxaximab, sold under the brand name Anthim among others, is a monoclonal antibody medication designed for the treatment of exposure to Bacillus anthracis spores.

Difelikefalin, sold under the brand name Korsuva, is an opioid peptide used for the treatment of moderate to severe itch. It acts as a peripherally-restricted, highly selective agonist of the κ-opioid receptor (KOR).
Eptinezumab, sold under the brand name Vyepti, is a medication used for the preventive treatment of migraine in adults. It is a monoclonal antibody that targets calcitonin gene-related peptides (CGRP) alpha and beta. It is administered by intravenous infusion.
Tagraxofusp, sold under the brand name Elzonris, is an anti-cancer medication for the treatment of blastic plasmacytoid dendritic cell neoplasm (BPDCN).
Fenofibrate/pravastatin, sold under the brand name Pravafenix, is a combination medication for the treatment of hypercholesterolemia in adults whose low-density lipoprotein (LDL) cholesterol is already being controlled with pravastatin alone but who still need to improve their cholesterol levels and to reduce their levels of triglycerides. It contains fenofibrate and pravastatin. It is taken by mouth.
Indacaterol/glycopyrronium bromide/mometasone, sold under the brand name Enerzair Breezhaler among others, is an inhalable fixed-dose combination medication for the treatment of asthma. It contains indacaterol as acetate, glycopyrronium bromide, and mometasone furoate.
Potassium citrate/potassium hydrogencarbonate is a fixed-dose combination medication intended for the treatment of distal renal tubular acidosis. It contains potassium citrate and potassium hydrogencarbonate.
Regdanvimab, sold under the brand name Regkirona, is a human monoclonal antibody used for the treatment of COVID-19. The antibody is directed against the spike protein of SARS-CoV-2. It is developed by Celltrion. The medicine is given by infusion (drip) into a vein.
Nivolumab/relatlimab, sold under the brand name Opdualag, is a fixed-dose combination medication use to treat melanoma. It contains nivolumab, a programmed death receptor-1 (PD-1) blocking antibody, and relatlimab, a lymphocyte activation gene-3 (LAG-3) blocking antibody. It is given by intravenous infusion.
Eladocagene exuparvovec, sold under the brand name Upstaza, is a gene therapy product for the treatment of aromatic L‑amino acid decarboxylase (AADC) deficiency. It infuses the gene encoding for the human AADC enzyme into the putamen region of the brain. The subsequent expression of AADC results in dopamine production and, as a result, development of motor function in patients with AADC deficiency.

Futibatinib, sold under the brand name Lytgobi, is an anti-cancer medication used for the treatment of cholangiocarcinoma. It is a kinase inhibitor. It is taken by mouth.

Gadopiclenol, sold under the brand name Elucirem among others, is a contrast agent used with magnetic resonance imaging (MRI) to detect and visualize lesions with abnormal vascularity in the central nervous system and in the body. Gadopiclenol is a paramagnetic macrocyclic non-ionic complex of gadolinium.
Cipaglucosidase alfa, sold under the brand name Pombiliti, and used in combination with miglustat, is a medication used for the treatment of glycogen storage disease type II. Cipaglucosidase alfa is a recombinant human acid α-glucosidase enzyme replacement therapy that provides an exogenous source of acid α-glucosidase.
Pegunigalsidase alfa, sold under the brand name Elfabrio, is an enzyme replacement therapy for the treatment of Fabry disease. It is a recombinant human α-galactosidase-A. It is a hydrolytic lysosomal neutral glycosphingolipid-specific enzyme.
Palopegteriparatide, sold under the brand name Yorvipath, is a hormone replacement therapy used for the treatment of chronic hypoparathyroidism. It is a transiently pegylated parathyroid hormone.

Arpraziquantel ((R)-praziquantel) is the eutomer (the biologically active enantiomer) of praziquantel, a medication which is under investigation for the treatment of schistosomiasis in young children with less side effects than the racemate.