 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | IV |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
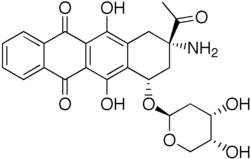
| Formula | C25H25NO9 |
| Molar mass | 483.473 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Amrubicin (INN; previously known as SM-5887) is an anthracycline used in the treatment of lung cancer. [1] It is marketed in Japan since 2002 by Sumitomo under the brand name Calsed. [2]
Amrubicin acts by inhibiting topoisomerase II, and has been compared in clinical trials with topotecan, a Topoisomerase I inhibitor. [3] [4]
It has also been studied for the treatment of bladder carcinoma [5] and gastric cancer. [6]
Amrubicin was the first anthracycline derivative created by de novo synthesis and was first published in 1989 by scientists from Sumitomo. [7]