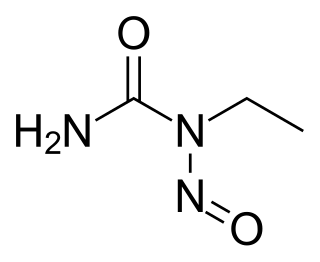
ENU, also known as N-ethyl-N-nitrosourea (chemical formula C3H7N3O2), is a highly potent mutagen. For a given gene in mice, ENU can induce 1 new mutation in every 700 loci. It is also toxic at high doses.

Diazomethane is the chemical compound CH2N2, discovered by German chemist Hans von Pechmann in 1894. It is the simplest diazo compound. In the pure form at room temperature, it is an extremely sensitive explosive yellow gas; thus, it is almost universally used as a solution in diethyl ether. The compound is a popular methylating agent in the laboratory, but it is too hazardous to be employed on an industrial scale without special precautions. Use of diazomethane has been significantly reduced by the introduction of the safer and equivalent reagent trimethylsilyldiazomethane.

Procarbazine is a chemotherapy medication used for the treatment of Hodgkin's lymphoma and brain cancers. For Hodgkin's it is often used together with chlormethine, vincristine, and prednisone while for brain cancers such as glioblastoma multiforme it is used with lomustine and vincristine. It is typically taken by mouth.

Losartan, sold under the trade name Cozaar among others, is a medication mainly used to treat high blood pressure. It is also used for diabetic kidney disease, heart failure, and left ventricular enlargement. It is taken by mouth. It may be used alone or in addition to other blood pressure medication. Up to six weeks may be required for the full effects to occur.
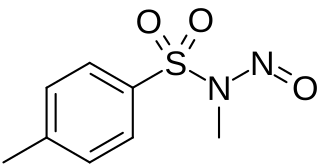
Diazald (N-methyl-N-nitroso-p-toluenesulfonamide) is used as a relatively safe and easily handled precursor to diazomethane, which is toxic and unstable. Diazald has become the favored commercially available precursor for the synthesis of diazomethane, compared to reagents like N-methyl-N-nitrosourea and N-methyl-N'-nitro-N-nitrosoguanidine, which are less thermally stable and more toxic and mutagenic, respectively.

Nitroso refers to a functional group in organic chemistry which has the NO group attached to an organic moiety. As such, various nitroso groups can be categorized as C-nitroso compounds (e.g., nitrosoalkanes; R−N=O), S-nitroso compounds (nitrosothiols; RS−N=O), N-nitroso compounds (e.g., nitrosamines, R1N(−R2)−N=O), and O-nitroso compounds (alkyl nitrites; RO−N=O).
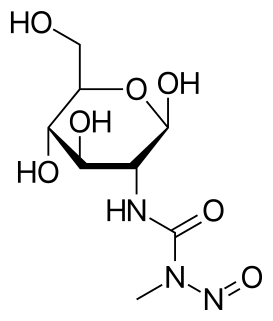
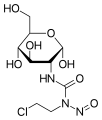
Streptozotocin or streptozocin (STZ) is a naturally occurring alkylating antineoplastic agent that is particularly toxic to the insulin-producing beta cells of the pancreas in mammals. It is used in medicine for treating certain cancers of the islets of Langerhans and used in medical research to produce an animal model for hyperglycemia and Alzheimer's in a large dose, as well as type 2 diabetes or type 1 diabetes with multiple low doses.

Nitrosobenzene is the organic compound with the formula C6H5NO. It is one of the prototypical organic nitroso compounds. Characteristic of its functional group, it is a dark green species that exists in equilibrium with its pale yellow dimer. Both monomer and dimer are diamagnetic.

Lomustine (INN); abbreviated as CCNU; original brand name CeeNU, now marketed as Gleostine) is an alkylating nitrosourea compound used in chemotherapy. It is closely related to semustine and is in the same family as streptozotocin. It is a highly lipid-soluble drug, thus it crosses the blood-brain barrier. This property makes it ideal for treating brain tumors, which is its primary use, although it is also used to treat Hodgkin lymphoma as a second-line option. Lomustine has a long time to nadir.
Carmustine, sold under the brand name BiCNU among others, is a medication used mainly for chemotherapy. It is a nitrogen mustard β-chloro-nitrosourea compound used as an alkylating agent.

Fotemustine is a nitrosourea alkylating agent used in the treatment of metastatic melanoma. It is available in Europe but has not been approved by the United States FDA. A study has shown that fotemustine produces improved response rates and but does not increase survival (over dacarbazine in the treatment of disseminated cutaneous melanoma. Median survival was 7.3 months with fotemustine versus 5.6 months with DTIC. There was also toxicity prevalence in fotemustine arm. The main toxicity was grade 3 to 4 neutropenia and thrombocytopenia.
2-Methyl-2-nitrosopropane (MNP or t-nitrosobutane) is the organic compound with the formula (CH3)3CNO. It is a blue liquid that is used in chemical research as a spin trap, i.e. it binds to radicals.
An alkylating antineoplastic agent is an alkylating agent used in cancer treatment that attaches an alkyl group (CnH2n+1) to DNA.

S-Nitrosothiols, also known as thionitrites, are organic compounds or functional groups containing a nitroso group attached to the sulfur atom of a thiol. S-Nitrosothiols have the general formula RSNO, where R denotes an organic group. Originally suggested by Ignarro to serve as intermediates in the action of organic nitrates, endogenous S-nitrosothiols were discovered by Stamler and colleagues and shown to represent a main source of NO bioactivity in vivo. More recently, S-nitrosothiols have been implicated as primary mediators of protein S-nitrosylation, the oxidative modification of Cys thiol that provides ubiquitous regulation of protein function.
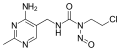
N-Nitroso-N-methylurea (NMU) is a highly reliable carcinogen, mutagen, and teratogen. NMU is an alkylating agent, and exhibits its toxicity by transferring its methyl group to nucleobases in nucleic acids, which can lead to AT:GC transition mutations.

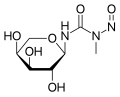
Arabinopyranosyl-N-methyl-N-nitrosourea, also known as Aranose (Араноза) is a cytostatic anticancer chemotherapeutic drug of an alkylating type. Chemically it is a nitrosourea derivative. It was developed in the Soviet Union in the 1970s. It was claimed by its developers that its advantages over other nitrosoureas are a relatively low hematological toxicity and a wider therapeutic index, which allows for its outpatient administration.

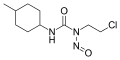
Nitrosamides are chemical compounds that contain of the chemical structure R1C(=X)N(–R2)–N=O, that is, a nitroso group bonded to the nitrogen of an amide or similar functional group. Specific classes include the N-nitrosamides, N-nitrosoureas, N-nitrosoguanidines, and N-nitrosocarbamates. Nitrosamides are usually chemically reactive, metabolically unstable, and often carcinogenic; however, in contrast to the N-nitrosamines, N-nitrosamides are not generally contaminants found in food.

Anaplastic oligodendroglioma is a neuroepithelial tumor which is believed to originate from oligodendrocytes, a cell type of the glia. In the World Health Organization (WHO) classification of brain tumors, anaplastic oligodendrogliomas are classified as grade III. In the course of the disease, they can degenerate into WHO grade IV glioblastoma. The vast majority of oligodendrogliomas occur sporadically, without a confirmed cause and without inheritance within a family.