 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Novantrone |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608019 |
| Routes of administration | Mainly intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | n/a |
| Protein binding | 78% |
| Metabolism | Hepatic (CYP2E1) |
| Elimination half-life | 75 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
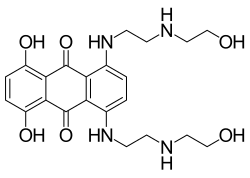
| Formula | C22H28N4O6 |
| Molar mass | 444.488 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Mitoxantrone (INN, BAN, USAN; also known as Mitozantrone in Australia; trade name Novantrone) is an anthracenedione antineoplastic agent.
