
Acute disseminated encephalomyelitis (ADEM), or acute demyelinating encephalomyelitis, is a rare autoimmune disease marked by a sudden, widespread attack of inflammation in the brain and spinal cord. As well as causing the brain and spinal cord to become inflamed, ADEM also attacks the nerves of the central nervous system and damages their myelin insulation, which, as a result, destroys the white matter. The cause is often a trigger such as from viral infection or vaccinations.
Multiplesclerosis (MS) is an autoimmune disease in which the insulating covers of nerve cells in the brain and spinal cord are damaged. This damage disrupts the ability of parts of the nervous system to transmit signals, resulting in a range of signs and symptoms, including physical, mental, and sometimes psychiatric problems. Specific symptoms can include double vision, vision loss, eye pain, muscle weakness, and loss of sensation or coordination. MS takes several forms, with new symptoms either occurring in isolated attacks or building up over time. In the relapsing forms of MS, between attacks, symptoms may disappear completely, although some permanent neurological problems often remain, especially as the disease advances. In the progressive forms of MS, bodily function slowly deteriorates and disability worsens once symptoms manifest and will steadily continue to do so if the disease is left untreated.
Neuromyelitis optica spectrum disorders (NMOSD), including neuromyelitis optica (NMO), are autoimmune diseases characterized by acute inflammation of the optic nerve and the spinal cord (myelitis). Episodes of ON and myelitis can be simultaneous or successive. A relapsing disease course is common, especially in untreated patients. In more than 80% of cases, NMO is caused by immunoglobulin G autoantibodies to aquaporin 4 (anti-AQP4), the most abundant water channel protein in the central nervous system. A subset of anti-AQP4-negative cases is associated with antibodies against myelin oligodendrocyte glycoprotein (anti-MOG). Rarely, NMO may occur in the context of other autoimmune diseases or infectious diseases. In some cases, the etiology remains unknown.
Interferon beta-1b is a cytokine in the interferon family used to treat the relapsing-remitting and secondary-progressive forms of multiple sclerosis (MS). It is approved for use after the first MS event. Closely related is interferon beta 1a, also indicated for MS, with a very similar drug profile.
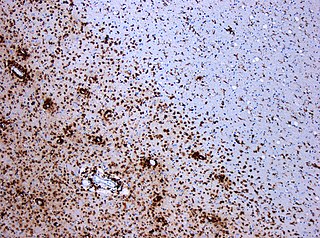
Multiple sclerosis and other demyelinating diseases of the central nervous system (CNS) produce lesions and glial scars or scleroses. They present different shapes and histological findings according to the underlying condition that produces them.
Inflammatory demyelinating diseases (IDDs), sometimes called Idiopathic (IIDDs) due to the unknown etiology of some of them, are a heterogenous group of demyelinating diseases - conditions that cause damage to myelin, the protective sheath of nerve fibers - that occur against the background of an acute or chronic inflammatory process. IDDs share characteristics with and are often grouped together under Multiple Sclerosis. They are sometimes considered different diseases from Multiple Sclerosis, but considered by others to form a spectrum differing only in terms of chronicity, severity, and clinical course.

Marburg acute multiple sclerosis, also known as Marburg multiple sclerosis or acute fulminant multiple sclerosis, is considered one of the multiple sclerosis borderline diseases, which is a collection of diseases classified by some as MS variants and by others as different diseases. Other diseases in this group are neuromyelitis optica (NMO), Balo concentric sclerosis, and Schilder's disease. The graver course is one form of malignant multiple sclerosis, with patients reaching a significant level of disability in less than five years from their first symptoms, often in a matter of months.

Laquinimod is an experimental immunomodulator developed by Active Biotech and Teva. It is being investigated as an oral treatment for multiple sclerosis (MS).
Research in multiple sclerosis may find new pathways to interact with the disease, improve function, curtail attacks, or limit the progression of the underlying disease. Many treatments already in clinical trials involve drugs that are used in other diseases or medications that have not been designed specifically for multiple sclerosis. There are also trials involving the combination of drugs that are already in use for multiple sclerosis. Finally, there are also many basic investigations that try to understand better the disease and in the future may help to find new treatments.
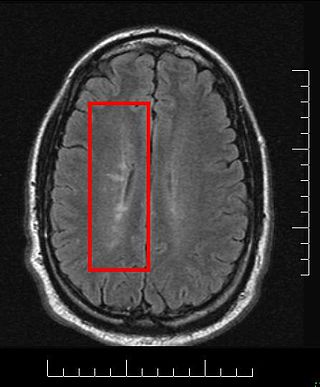
A clinically isolated syndrome (CIS) is a clinical situation of an individual's first neurological episode, caused by inflammation or demyelination of nerve tissue. An episode may be monofocal, in which symptoms present at a single site in the central nervous system, or multifocal, in which multiple sites exhibit symptoms. CIS with enough paraclinical evidence can be considered as a clinical stage of multiple sclerosis (MS). It can also be retrospectively diagnosed as a kind of MS when more evidence is available.

Tumefactive multiple sclerosis is a condition in which the central nervous system of a person has multiple demyelinating lesions with atypical characteristics for those of standard multiple sclerosis (MS). It is called tumefactive as the lesions are "tumor-like" and they mimic tumors clinically, radiologically and sometimes pathologically.
Poser criteria are diagnostic criteria for multiple sclerosis (MS). They replaced the older Schumacher criteria, and are now considered obsolete as McDonald criteria have superseded them. Nevertheless, some of the concepts introduced have remained in MS research, like CDMS, and newer criteria are often calibrated against them. The criteria were unveiled in the Annals of Neurology in 1983 by a team led by Dr. Charles M. Poser.
Schumacher criteria are diagnostic criteria that were previously used for identifying multiple sclerosis (MS). Multiple sclerosis, understood as a central nervous system (CNS) condition, can be difficult to diagnose since its signs and symptoms may be similar to other medical problems. Medical organizations have created diagnostic criteria to ease and standardize the diagnostic process especially in the first stages of the disease. Schumacher criteria were the first internationally recognized criteria for diagnosis, and introduced concepts still in use, as CDMS.
Malignant multiple sclerosis is used to describe MS patients who reach significant level of disability in a short period of time. Malignant MS cases are not common, less than 5% of patients with MS experience this type of progression.

Current standards for diagnosing multiple sclerosis (MS) are based on the 2018 revision of McDonald criteria. They rely on MRI detection of demyelinating lesions in the CNS, which are distributed in space (DIS) and in time (DIT). It is also a requirement that any possible known disease that produces demyelinating lesions is ruled out before applying McDonald's criteria.
Clinical Electrophysiological Testing is based on techniques derived from electrophysiology used for the clinical diagnosis of patients. There are many processes that occur in the body which produce electrical signals that can be detected. Depending on the location and the source of these signals, distinct methods and techniques have been developed to properly target them.

Multiple sclerosis (MS) can be pathologically defined as the presence of distributed glial scars (scleroses) in the central nervous system that must show dissemination in time (DIT) and in space (DIS) to be considered MS lesions.
Radiologically isolated syndrome (RIS) is a clinical situation in which a person has white matter lesions suggestive of multiple sclerosis (MS), as shown on an MRI scan that was done for reasons unrelated to MS symptoms. The nerve lesions in these people show dissemination in space with an otherwise normal neurological examination and without historical accounts of typical MS symptoms.
Anti-AQP4 diseases, are a group of diseases characterized by auto-antibodies against aquaporin 4.
Brenda Banwell is Chief of the Division of Neurology and Co-Director of the Neuroscience Center, and Professor of Neurology at Children's Hospital of Philadelphia and holder of the Grace R. Loeb Endowed Chair in Neurosciences. She also holds the title of Professor of Pediatrics and Neurology at the Perelman School of Medicine at the University of Pennsylvania.








