Chronic lymphocytic leukemia (CLL) is a type of cancer in which the bone marrow makes too many lymphocytes. Early on, there are typically no symptoms. Later, non-painful lymph node swelling, feeling tired, fever, night sweats, or weight loss for no clear reason may occur. Enlargement of the spleen and low red blood cells (anemia) may also occur. It typically worsens gradually over years.

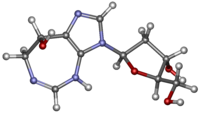
Cytarabine, also known as cytosine arabinoside (ara-C), is a chemotherapy medication used to treat acute myeloid leukemia (AML), acute lymphocytic leukemia (ALL), chronic myelogenous leukemia (CML), and non-Hodgkin's lymphoma. It is given by injection into a vein, under the skin, or into the cerebrospinal fluid. There is a liposomal formulation for which there is tentative evidence of better outcomes in lymphoma involving the meninges.

Adenosine deaminase is an enzyme involved in purine metabolism. It is needed for the breakdown of adenosine from food and for the turnover of nucleic acids in tissues.

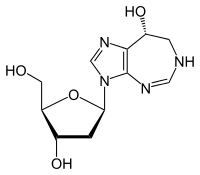
Cladribine, sold under the brand name Leustatin, among others, is a medication used to treat hairy cell leukemia and B-cell chronic lymphocytic leukemia. Cladribine, sold under the brand name Mavenclad, is used for the treatment of adults with highly active forms of relapsing-remitting multiple sclerosis.

Fludarabine is a purine analogue and antineoplastic agent. It is generally used as its 5-O-phosphorylated form known as fludarabine phosphate, sold under the brand name Fludara among others. It is a chemotherapy medication used in the treatment of leukemia and lymphoma. These include chronic lymphocytic leukemia, non-Hodgkin's lymphoma, acute myeloid leukemia, and acute lymphocytic leukemia. It is given by injection into a vein or by mouth.

Bruton's tyrosine kinase, also known as tyrosine-protein kinase BTK, is a tyrosine kinase that is encoded by the BTK gene in humans. BTK plays a crucial role in B cell development.

Deoxyadenosine triphosphate (dATP) is a nucleotide used in cells for DNA synthesis, as a substrate of DNA polymerase.
Lumiliximab is an IgG1k monoclonal antibody that targets CD23. It acts as an immunomodulator and was awarded orphan drug status and fast track designation by the FDA.

Bendamustine, sold under the brand name Treanda among others, is a chemotherapy medication used in the treatment of chronic lymphocytic leukemia (CLL), multiple myeloma, and non-Hodgkin's lymphoma. It is given by injection into a vein.
Oblimersen is an antisense oligodeoxyribonucleotide being studied as a possible treatment for several types of cancer, including chronic lymphocytic leukemia, B-cell lymphoma, and breast cancer. It may kill cancer cells by blocking the production of Bcl-2—a protein that makes cancer cells live longer—and by making them more sensitive to chemotherapy.

Omacetaxine mepesuccinate is a pharmaceutical drug substance that is indicated for treatment of chronic myeloid leukemia (CML).
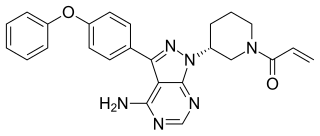
Ibrutinib, sold under the brand name Imbruvica among others, is a small molecule drug that inhibits B-cell proliferation and survival by irreversibly binding the protein Bruton's tyrosine kinase (BTK). Blocking BTK inhibits the B-cell receptor pathway, which is often aberrantly active in B cell cancers. Ibrutinib is therefore used to treat such cancers, including mantle cell lymphoma, chronic lymphocytic leukemia, and Waldenström's macroglobulinemia. Ibrutinib also binds to C-terminal Src Kinases. These are off-target receptors for the BTK inhibitor. Ibrutinib binds to these receptors and inhibits the kinase from promoting cell differentiation and growth. This leads to many different side effects like left atrial enlargement and atrial fibrillation during the treatment of Chronic Lymphocytic Leukemia.
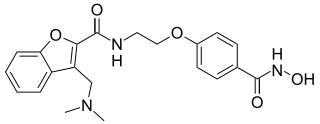
Abexinostat is an experimental drug candidate for cancer treatment. It was developed by Pharmacyclics and licensed to Xynomic. As of 2013, it was in Phase II clinical trials for B-cell lymphoma. Pre-clinical study suggests the potential for treatment of different types of cancer as well.

Idelalisib, sold under the brand name Zydelig, is a medication used to treat certain blood cancers. Idelalisib acts as a phosphoinositide 3-kinase inhibitor; more specifically, it blocks P110δ, the delta isoform of the enzyme phosphoinositide 3-kinase. It was developed by Gilead Sciences. It is taken orally.
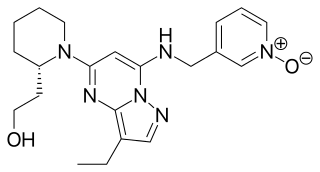
Dinaciclib (SCH-727965) is an experimental drug that inhibits cyclin-dependent kinases (CDKs). It is being evaluated in clinical trials for various cancer indications.

Acalabrutinib, sold under the brand name Calquence, is a medication used to treat various types of non-Hodgkin lymphoma, including mantle cell lymphoma (MCL) and chronic lymphocytic leukemia/small lymphocytic Lymphoma (CLL/SLL). It may be used both in relapsed as well as in treatment-naive settings.

Duvelisib, sold under the brand name Copiktra, is a medication used to treat chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL), and follicular lymphoma after other treatments have failed. It is taken by mouth. It is a PI3 kinase inhibitor.
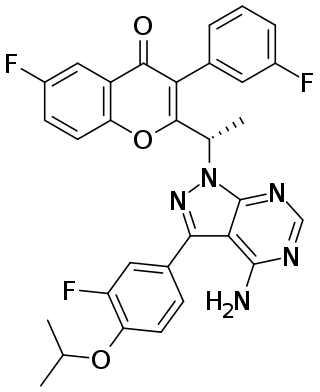
Umbralisib, sold under the brand name Ukoniq, is an anti-cancer medication for the treatment of marginal zone lymphoma (MZL) and follicular lymphoma (FL). It is taken by mouth.
Verastem Oncology is an American pharmaceutical company that develops medicines to treat certain cancers. Headquartered and founded in Boston, Massachusetts, the firm is a member of NASDAQ Biotechnology Index.

Pirtobrutinib, sold under the brand name Jaypirca, is an anticancer medication that is used to treat mantle cell lymphoma. It inhibits B cell lymphocyte proliferation and survival by binding and inhibiting Bruton's tyrosine kinase (BTK). It is taken by mouth.