
Bacillus is a genus of Gram-positive, rod-shaped bacteria, a member of the phylum Bacillota, with 266 named species. The term is also used to describe the shape (rod) of other so-shaped bacteria; and the plural Bacilli is the name of the class of bacteria to which this genus belongs. Bacillus species can be either obligate aerobes which are dependent on oxygen, or facultative anaerobes which can survive in the absence of oxygen. Cultured Bacillus species test positive for the enzyme catalase if oxygen has been used or is present.

Agrobacterium tumefaciens is the causal agent of crown gall disease in over 140 species of eudicots. It is a rod-shaped, Gram-negative soil bacterium. Symptoms are caused by the insertion of a small segment of DNA, from a plasmid into the plant cell, which is incorporated at a semi-random location into the plant genome. Plant genomes can be engineered by use of Agrobacterium for the delivery of sequences hosted in T-DNA binary vectors.

Bacillus subtilis, known also as the hay bacillus or grass bacillus, is a gram-positive, catalase-positive bacterium, found in soil and the gastrointestinal tract of ruminants, humans and marine sponges. As a member of the genus Bacillus, B. subtilis is rod-shaped, and can form a tough, protective endospore, allowing it to tolerate extreme environmental conditions. B. subtilis has historically been classified as an obligate aerobe, though evidence exists that it is a facultative anaerobe. B. subtilis is considered the best studied Gram-positive bacterium and a model organism to study bacterial chromosome replication and cell differentiation. It is one of the bacterial champions in secreted enzyme production and used on an industrial scale by biotechnology companies.

Mycobacterium smegmatis is an acid-fast bacterial species in the phylum Actinomycetota and the genus Mycobacterium. It is 3.0 to 5.0 μm long with a bacillus shape and can be stained by Ziehl–Neelsen method and the auramine-rhodamine fluorescent method. It was first reported in November 1884, who found a bacillus with the staining appearance of tubercle bacilli in syphilitic chancres. Subsequent to this, Alvarez and Tavel found organisms similar to that described by Lustgarten also in normal genital secretions (smegma). This organism was later named M. smegmatis.

Lacticaseibacillus casei is an organism that belongs to the largest genus in the family Lactobacillaceae, a lactic acid bacteria (LAB), that was previously classified as Lactobacillus casei. This bacteria has been identified as facultatively anaerobic or microaerophilic, acid-tolerant, non-spore-forming bacteria.

The avermectins are a group of 16-membered macrocyclic lactone derivatives with potent anthelmintic and insecticidal properties. These naturally occurring compounds are generated as fermentation products by Streptomyces avermitilis, a soil actinomycete. Eight different avermectins were isolated in four pairs of homologue compounds, with a major (a-component) and minor (b-component) component usually in ratios of 80:20 to 90:10. Avermectin B1, a mixture of B1a and B1b, is the drug and pesticide abamectin. Other anthelmintics derived from the avermectins include ivermectin, selamectin, doramectin, eprinomectin.
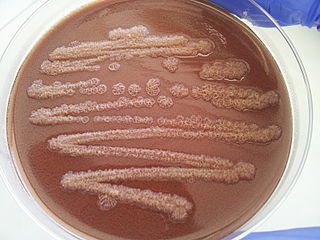
Pseudomonas stutzeri is a Gram-negative soil bacterium that is motile, has a single polar flagellum, and is classified as bacillus, or rod-shaped. While this bacterium was first isolated from human spinal fluid, it has since been found in many different environments due to its various characteristics and metabolic capabilities. P. stutzeri is an opportunistic pathogen in clinical settings, although infections are rare. Based on 16S rRNA analysis, this bacterium has been placed in the P. stutzeri group, to which it lends its name.

Aminocoumarin is a class of antibiotics that act by an inhibition of the DNA gyrase enzyme involved in the cell division in bacteria. They are derived from Streptomyces species, whose best-known representative – Streptomyces coelicolor – was completely sequenced in 2002. The aminocoumarin antibiotics include:

Streptomyces griseus is a species of bacteria in the genus Streptomyces commonly found in soil. A few strains have been also reported from deep-sea sediments. It is a Gram-positive bacterium with high GC content. Along with most other streptomycetes, S. griseus strains are well known producers of antibiotics and other such commercially significant secondary metabolites. These strains are known to be producers of 32 different structural types of bioactive compounds. Streptomycin, the first antibiotic ever reported from a bacterium, comes from strains of S. griseus. Recently, the whole genome sequence of one of its strains had been completed.
Streptomyces scabiei is a streptomycete bacterium species found in soils around the world. Unlike most of the 500 or so Streptomyces species it is a plant pathogen causing corky lesions to form on tuber and root crops as well as decreasing the growth of seedlings. Along with other closely related species it causes the potato disease common scab, which is an economically important disease in many potato growing areas. It was first described in 1892, being classified as a fungus, before being renamed in 1914 and again in 1948. Several other species of Streptomyces cause similar diseases to S. scabiei but other, more closely related species, do not.

Streptomyces avermitilis is a species of bacteria in the genus Streptomyces. This bacterium was discovered by Satoshi Ōmura in Shizuoka Prefecture, Japan.

Penicillium rubens is a species of fungus in the genus Penicillium and was the first species known to produce the antibiotic penicillin. It was first described by Philibert Melchior Joseph Ehi Biourge in 1923. For the discovery of penicillin from this species Alexander Fleming shared the Nobel Prize in Physiology or Medicine in 1945. The original penicillin-producing type has been variously identified as Penicillium rubrum, P. notatum, and P. chrysogenum among others, but genomic comparison and phylogenetic analysis in 2011 resolved that it is P. rubens. It is the best source of penicillins and produces benzylpenicillin (G), phenoxymethylpenicillin (V) and octanoylpenicillin (K). It also produces other important bioactive compounds such as andrastin, chrysogine, fungisporin, roquefortine, and sorbicillins.
Streptomyces isolates have yielded the majority of human, animal, and agricultural antibiotics, as well as a number of fundamental chemotherapy medicines. Streptomyces is the largest antibiotic-producing genus of Actinomycetota, producing chemotherapy, antibacterial, antifungal, antiparasitic drugs, and immunosuppressants. Streptomyces isolates are typically initiated with the aerial hyphal formation from the mycelium.
Mervyn James Bibb is an Emeritus Fellow at the John Innes Centre, Norwich, UK.

Streptomyces antibioticus is a gram-positive bacterium discovered in 1941 by Nobel-prize-winner Selman Waksman and H. Boyd Woodruff. Its name is derived from the Greek "strepto-" meaning "twisted", alluding to this genus' chain-like spore production, and "antibioticus", referring to this species' extensive antibiotic production. Upon its first characterization, it was noted that S. antibioticus produces a distinct soil odor.
Streptomyces albidoflavus is a bacterium species from the genus of Streptomyces which has been isolated from soil from Poland. Streptomyces albidoflavus produces dibutyl phthalate and streptothricins.
Streptomyces albofaciens is a bacterium species from the genus of Streptomyces which produces oxytetracycline, spiramycin, albopeptin A, albopeptin B and alpomycin.
Streptomyces lavendulae is a species of bacteria from the genus Streptomyces. It is isolated from soils globally and is known for its production of medically useful biologically active metabolites. To see a photo of this organism click here.
Streptomyces glaucescens is a bacterium species from the genus of Streptomyces which has been isolated from soil. Streptomyces glaucescens produces tetracenomycin C, tetracenomycin D and tetracenomycin E.
Streptomyces sanglieri is a bacterium species from the genus of Streptomyces which has been isolated from soil from a hay meadow. Streptomyces sanglieri produces the antibiotic lactonamycin Z.














