Peptidoglycan or murein is a unique large macromolecule, a polysaccharide, consisting of sugars and amino acids that forms a mesh-like peptidoglycan layer (sacculus) that surrounds the bacterial cytoplasmic membrane. The sugar component consists of alternating residues of β-(1,4) linked N-acetylglucosamine (NAG) and N-acetylmuramic acid (NAM). Attached to the N-acetylmuramic acid is an oligopeptide chain made of three to five amino acids. The peptide chain can be cross-linked to the peptide chain of another strand forming the 3D mesh-like layer. Peptidoglycan serves a structural role in the bacterial cell wall, giving structural strength, as well as counteracting the osmotic pressure of the cytoplasm. This repetitive linking results in a dense peptidoglycan layer which is critical for maintaining cell form and withstanding high osmotic pressures, and it is regularly replaced by peptidoglycan production. Peptidoglycan hydrolysis and synthesis are two processes that must occur in order for cells to grow and multiply, a technique carried out in three stages: clipping of current material, insertion of new material, and re-crosslinking of existing material to new material.
In enzymology, an UDP-N-acetylglucosamine 6-dehydrogenase (EC 1.1.1.136) is an enzyme that catalyzes the chemical reaction

In enzymology, an UDP-N-acetylmuramate dehydrogenase (EC 1.3.1.98) is an enzyme that catalyzes the chemical reaction

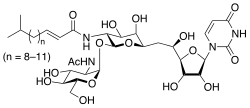
Uridine diphosphate N-acetylglucosamine or UDP-GlcNAc is a nucleotide sugar and a coenzyme in metabolism. It is used by glycosyltransferases to transfer N-acetylglucosamine residues to substrates. D-Glucosamine is made naturally in the form of glucosamine-6-phosphate, and is the biochemical precursor of all nitrogen-containing sugars. To be specific, glucosamine-6-phosphate is synthesized from fructose 6-phosphate and glutamine as the first step of the hexosamine biosynthesis pathway. The end-product of this pathway is UDP-GlcNAc, which is then used for making glycosaminoglycans, proteoglycans, and glycolipids.
The enzyme UDP-glucose 4-epimerase, also known as UDP-galactose 4-epimerase or GALE, is a homodimeric epimerase found in bacterial, fungal, plant, and mammalian cells. This enzyme performs the final step in the Leloir pathway of galactose metabolism, catalyzing the reversible conversion of UDP-galactose to UDP-glucose. GALE tightly binds nicotinamide adenine dinucleotide (NAD+), a co-factor required for catalytic activity.
In enzymology, an UDP-N-acetylglucosamine 2-epimerase is an enzyme that catalyzes the chemical reaction
Nucleotide sugars are the activated forms of monosaccharides. Nucleotide sugars act as glycosyl donors in glycosylation reactions. Those reactions are catalyzed by a group of enzymes called glycosyltransferases.

In enzymology, N-acetylglucosamine-6-phosphate deacetylase (EC 3.5.1.25), also known as GlcNAc-6-phosphate deacetylase or NagA, is an enzyme that catalyzes the deacetylation of N-acetylglucosamine-6-phosphate (GlcNAc-6-P) to glucosamine-6-phosphate (GlcN-6-P):

In enzymology, an UDP-N-acetylglucosamine 1-carboxyvinyltransferase is an enzyme that catalyzes the first committed step in peptidoglycan biosynthesis of bacteria:
In enzymology, a N-acetyllactosaminide 3-alpha-galactosyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a N-acetyllactosaminide beta-1,3-N-acetylglucosaminyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a protein N-acetylglucosaminyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, an UDP-N-acetylglucosamine—dolichyl-phosphate N-acetylglucosaminephosphotransferase is an enzyme that catalyzes the chemical reaction

In enzymology, an UDP-N-acetylglucosamine diphosphorylase is an enzyme that catalyzes the chemical reaction

Polypeptide N-acetylgalactosaminyltransferase 3 is an enzyme that in humans is encoded by the GALNT3 gene.

UDP-N-acetylglucosamine—dolichyl-phosphate N-acetylglucosaminephosphotransferase is an enzyme that in humans is encoded by the DPAGT1 gene.

Polypeptide N-acetylgalactosaminyltransferase 2 is an enzyme that in humans is encoded by the GALNT2 gene.

Ribostamycin is an aminoglycoside-aminocyclitol antibiotic isolated from a streptomycete, Streptomyces ribosidificus, originally identified in a soil sample from Tsu City of Mie Prefecture in Japan. It is made up of 3 ring subunits: 2-deoxystreptamine (DOS), neosamine C, and ribose. Ribostamycin, along with other aminoglycosides with the DOS subunit, is an important broad-spectrum antibiotic with important use against human immunodeficiency virus and is considered a critically important antimicrobial by the World Health Organization., Resistance against aminoglycoside antibiotics, such as ribostamycin, is a growing concern. The resistant bacteria contain enzymes that modify the structure through phosphorylation, adenylation, and acetylation and prevent the antibiotic from being able to interact with the bacterial ribosomal RNAs.
O-linked glycosylation is the attachment of a sugar molecule to the oxygen atom of serine (Ser) or threonine (Thr) residues in a protein. O-glycosylation is a post-translational modification that occurs after the protein has been synthesised. In eukaryotes, it occurs in the endoplasmic reticulum, Golgi apparatus and occasionally in the cytoplasm; in prokaryotes, it occurs in the cytoplasm. Several different sugars can be added to the serine or threonine, and they affect the protein in different ways by changing protein stability and regulating protein activity. O-glycans, which are the sugars added to the serine or threonine, have numerous functions throughout the body, including trafficking of cells in the immune system, allowing recognition of foreign material, controlling cell metabolism and providing cartilage and tendon flexibility. Because of the many functions they have, changes in O-glycosylation are important in many diseases including cancer, diabetes and Alzheimer's. O-glycosylation occurs in all domains of life, including eukaryotes, archaea and a number of pathogenic bacteria including Burkholderia cenocepacia, Neisseria gonorrhoeae and Acinetobacter baumannii.

Protein O-GlcNAc transferase also known as OGT or O-linked N-acetylglucosaminyltransferase is an enzyme that in humans is encoded by the OGT gene. OGT catalyzes the addition of the O-GlcNAc post-translational modification to proteins.