
Beta-lactamases, (β-lactamases) are enzymes produced by bacteria that provide multi-resistance to beta-lactam antibiotics such as penicillins, cephalosporins, cephamycins, monobactams and carbapenems (ertapenem), although carbapenems are relatively resistant to beta-lactamase. Beta-lactamase provides antibiotic resistance by breaking the antibiotics' structure. These antibiotics all have a common element in their molecular structure: a four-atom ring known as a beta-lactam (β-lactam) ring. Through hydrolysis, the enzyme lactamase breaks the β-lactam ring open, deactivating the molecule's antibacterial properties.
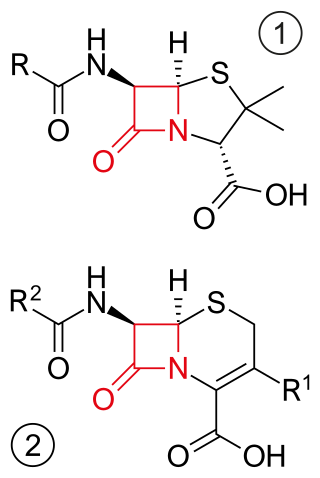
β-lactam antibiotics are antibiotics that contain a beta-lactam ring in their chemical structure. This includes penicillin derivatives (penams), cephalosporins and cephamycins (cephems), monobactams, carbapenems and carbacephems. Most β-lactam antibiotics work by inhibiting cell wall biosynthesis in the bacterial organism and are the most widely used group of antibiotics. Until 2003, when measured by sales, more than half of all commercially available antibiotics in use were β-lactam compounds. The first β-lactam antibiotic discovered, penicillin, was isolated from a strain of Penicillium rubens.
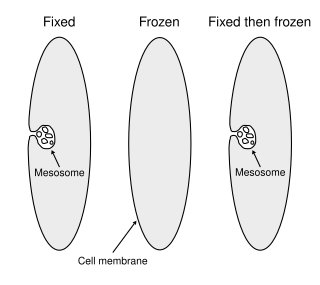
Mesosomes or chondrioids are folded invaginations in the plasma membrane of bacteria that are produced by the chemical fixation techniques used to prepare samples for electron microscopy. Although several functions were proposed for these structures in the 1960s, they were recognized as artifacts by the late 1970s and are no longer considered to be part of the normal structure of bacterial cells. These extensions are in the form of vesicles, tubules and lamellae.

Cephamycins are a group of β-lactam antibiotics. They are very similar to cephalosporins, and the cephamycins are sometimes classified as cephalosporins.

Cephems are a sub-group of β-lactam antibiotics including cephalosporins and cephamycins. It is one of the most common 4-membered ring heterocycle. Produced by actinomycetes, cephamycins were found to display antibacterial activity against a wide range of bacteria, including those resistant to penicillin and cephalosporins. The antimicrobial properties of Cephem include the attachment to certain penicillin-binding proteins that are involved in the production of cell walls of bacteria.

Clavulanic acid is a β-lactam drug that functions as a mechanism-based β-lactamase inhibitor. While not effective by itself as an antibiotic, when combined with penicillin-group antibiotics, it can overcome antibiotic resistance in bacteria that secrete β-lactamase, which otherwise inactivates most penicillins.

Carbapenems are a class of very effective antibiotic agents most commonly used for the treatment of severe bacterial infections. This class of antibiotics is usually reserved for known or suspected multidrug-resistant (MDR) bacterial infections. Similar to penicillins and cephalosporins, carbapenems are members of the beta lactam class of antibiotics, which kill bacteria by binding to penicillin-binding proteins, thus inhibiting bacterial cell wall synthesis. However, these agents individually exhibit a broader spectrum of activity compared to most cephalosporins and penicillins. Furthermore, carbapenems are typically unaffected by emerging antibiotic resistance, even to other beta-lactams.

Temocillin is a β-lactamase-resistant penicillin introduced by Beecham, marketed by Eumedica Pharmaceuticals as Negaban. It is used primarily for the treatment of multiple drug-resistant, Gram-negative bacteria.
It is a 6-methoxy penicillin; it is also a carboxypenicillin.

Faropenem is an orally active beta-lactam antibiotic belonging to the penem group. It is resistant to some forms of extended-spectrum beta-lactamase. It is available for oral use.

Cefoxitin is a second-generation cephamycin antibiotic developed by Merck & Co., Inc. from Cephamycin C in the year following its discovery, 1972. It was synthesized in order to create an antibiotic with a broader spectrum. It is often grouped with the second-generation cephalosporins. Cefoxitin requires a prescription and as of 2010 is sold under the brand name Mefoxin by Bioniche Pharma, LLC. The generic version of cefoxitin is known as cefoxitin sodium.
Capnocytophaga is a genus of Gram-negative bacteria. Normally found in the oropharyngeal tract of mammals, they are involved in the pathogenesis of some animal bite wounds and periodontal diseases.
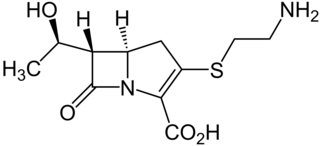
Thienamycin is one of the most potent naturally produced antibiotics known thus far, discovered in Streptomyces cattleya in 1976. Thienamycin has excellent activity against both Gram-positive and Gram-negative bacteria and is resistant to bacterial β-lactamase enzymes. Thienamycin is a zwitterion at pH 7.

Beta-lactamases are a family of enzymes involved in bacterial resistance to beta-lactam antibiotics. In bacterial resistance to beta-lactam antibiotics, the bacteria have beta-lactamase which degrade the beta-lactam rings, rendering the antibiotic ineffective. However, with beta-lactamase inhibitors, these enzymes on the bacteria are inhibited, thus allowing the antibiotic to take effect. Strategies for combating this form of resistance have included the development of new beta-lactam antibiotics that are more resistant to cleavage and the development of the class of enzyme inhibitors called beta-lactamase inhibitors. Although β-lactamase inhibitors have little antibiotic activity of their own, they prevent bacterial degradation of beta-lactam antibiotics and thus extend the range of bacteria the drugs are effective against.

Aminocoumarin is a class of antibiotics that act by an inhibition of the DNA gyrase enzyme involved in the cell division in bacteria. They are derived from Streptomyces species, whose best-known representative – Streptomyces coelicolor – was completely sequenced in 2002. The aminocoumarin antibiotics include:

Kingella kingae is a species of Gram-negative facultative anaerobic β-hemolytic coccobacilli. First isolated in 1960 by Elizabeth O. King, it was not recognized as a significant cause of infection in young children until the 1990s, when culture techniques had improved enough for it to be recognized. It is best known as a cause of septic arthritis, osteomyelitis, spondylodiscitis, bacteraemia, and endocarditis, and less frequently lower respiratory tract infections and meningitis.

Clavams are a class of antibiotics. This antibiotic is derived from Streptomyces clavuligerus NRRL 3585. Clavam is produced to form a new β-lactam antibiotic. This class is divided into the clavulanic acid class and the 5S clavams class. Clavulanic acid is a broad-spectrum antibiotic and 5S clavams may have anti-fungal properties. They are similar to penams, but with an oxygen substituted for the sulfur. Thus, they are also known as oxapenams.

Proteus penneri is a Gram-negative, facultatively anaerobic, rod-shaped bacterium. It is an invasive pathogen and a cause of nosocomial infections of the urinary tract or open wounds. Pathogens have been isolated mainly from the urine of patients with abnormalities in the urinary tract, and from stool. P. penneri strains are naturally resistant to numerous antibiotics, including penicillin G, amoxicillin, cephalosporins, oxacillin, and most macrolides, but are naturally sensitive to aminoglycosides, carbapenems, aztreonam, quinolones, sulphamethoxazole, and co-trimoxazole. Isolates of P. penneri have been found to be multiple drug-resistant (MDR) with resistance to six to eight drugs. β-lactamase production has also been identified in some isolates.

Avibactam is a non-β-lactam β-lactamase inhibitor developed by Actavis jointly with AstraZeneca. A new drug application for avibactam in combination with ceftazidime was approved by the FDA on February 25, 2015, for treating complicated urinary tract (cUTI) and complicated intra-abdominal infections (cIAI) caused by antibiotic resistant-pathogens, including those caused by multi-drug resistant Gram-negative bacterial pathogens.

Ceftazidime/avibactam, sold under the brand name Avycaz among others, is a fixed-dose combination medication composed of ceftazidime, a cephalosporin antibiotic, and avibactam, a β-lactamase inhibitor. It is used to treat complicated intra-abdominal infections, urinary tract infections, and pneumonia. It is only recommended when other options are not appropriate. It is given by injection into a vein.

Karen Bush is an American biochemist. She is a Professor of Practice in Biology at Indiana University and the interim director of the Biotechnology program. Bush conducts research focusing on bacterial resistance mechanisms to beta-lactam antibiotics.
















