| Campylobacterota | |
|---|---|
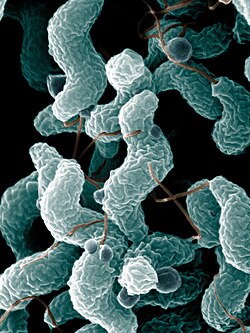 | |
| Campylobacter | |
| Scientific classification | |
| Domain: | Bacteria |
| Kingdom: | Pseudomonadati |
| Phylum: | Campylobacterota Waite et al. 2021 [1] |
| Classes | |
| Synonyms | |
| |
Campylobacterota are a phylum of Gram-negative bacteria. [3]
| Campylobacterota | |
|---|---|
 | |
| Campylobacter | |
| Scientific classification | |
| Domain: | Bacteria |
| Kingdom: | Pseudomonadati |
| Phylum: | Campylobacterota Waite et al. 2021 [1] |
| Classes | |
| Synonyms | |
| |
Campylobacterota are a phylum of Gram-negative bacteria. [3]
Until the 2021 revision of bacterial taxonomy by the ICSP, [4] the entire phylum was classified within the Proteobacteria (synonym Pseudomonadota) as the Epsilonproteobacteria and the Desulfurellales. [5] The separation of this phylum from "Proteobacteria" was originally proposed in 2017, using GTDB-based methods. [2]
The currently accepted taxonomy is based on the List of Prokaryotic names with Standing in Nomenclature (LPSN) [6] and National Center for Biotechnology Information (NCBI). [7]
| 16S rRNA based LTP_10_2024 [8] [9] [10] | 120 marker proteins based GTDB 10-RS226 [11] [12] [13] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|