
Rickettsia is a genus of nonmotile, gram-negative, nonspore-forming, highly pleomorphic bacteria that may occur in the forms of cocci, bacilli, or threads. The genus was named after Howard Taylor Ricketts in honor of his pioneering work on tick-borne spotted fever.
Rickettsia prowazekii is a species of gram-negative, alphaproteobacteria, obligate intracellular parasitic, aerobic bacillus bacteria that is the etiologic agent of epidemic typhus, transmitted in the feces of lice. In North America, the main reservoir for R. prowazekii is the flying squirrel. R. prowazekii is often surrounded by a protein microcapsular layer and slime layer; the natural life cycle of the bacterium generally involves a vertebrate and an invertebrate host, usually an arthropod, typically the human body louse. A form of R. prowazekii that exists in the feces of arthropods remains stably infective for months. R. prowazekii also appears to be the closest free-living relative of mitochondria, based on genome sequencing.

The Rickettsiales, informally called rickettsias, are an order of small Alphaproteobacteria. They are obligate intracellular parasites, and some are notable pathogens, including Rickettsia, which causes a variety of diseases in humans, and Ehrlichia, which causes diseases in livestock. Another genus of well-known Rickettsiales is the Wolbachia, which infect about two-thirds of all arthropods and nearly all filarial nematodes. Genetic studies support the endosymbiotic theory according to which mitochondria and related organelles developed from members of this group.

Rickettsia rickettsii is a Gram-negative, intracellular, coccobacillus bacterium that was first discovered in 1902. R. rickettsii is the causative agent of Rocky Mountain Spotted Fever and is transferred to its host via a tick bite. It is one of the most pathogenic Rickettsia species and affects a large majority of the Western Hemisphere, most commonly the Americas.
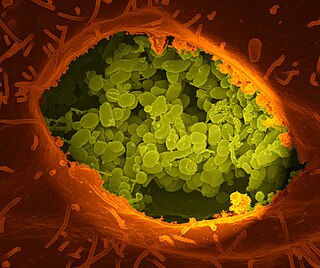
Coxiella burnetii is an obligate intracellular bacterial pathogen, and is the causative agent of Q fever. The genus Coxiella is morphologically similar to Rickettsia, but with a variety of genetic and physiological differences. C. burnetii is a small Gram-negative, coccobacillary bacterium that is highly resistant to environmental stresses such as high temperature, osmotic pressure, and ultraviolet light. These characteristics are attributed to a small cell variant form of the organism that is part of a biphasic developmental cycle, including a more metabolically and replicatively active large cell variant form. It can survive standard disinfectants, and is resistant to many other environmental changes like those presented in the phagolysosome.

Genome size is the total amount of DNA contained within one copy of a single complete genome. It is typically measured in terms of mass in picograms or less frequently in daltons, or as the total number of nucleotide base pairs, usually in megabases. One picogram is equal to 978 megabases. In diploid organisms, genome size is often used interchangeably with the term C-value.
The Weil–Felix test is an agglutination test for the diagnosis of rickettsial infections. It was first described in 1916. By virtue of its long history and of its simplicity, it has been one of the most widely employed tests for rickettsia on a global scale, despite being superseded in many settings by more sensitive and specific diagnostic tests. The Weil-Felix antibody was recently found to target rickettsia LPS O-antigen.

Meningoencephalitis, also known as herpes meningoencephalitis, is a medical condition that simultaneously resembles both meningitis, which is an infection or inflammation of the meninges, and encephalitis, which is an infection or inflammation of the brain tissue.

Orientia tsutsugamushi is a mite-borne bacterium belonging to the family Rickettsiaceae and is responsible for a disease called scrub typhus in humans. It is a natural and an obligate intracellular parasite of mites belonging to the family Trombiculidae. With a genome of only 2.0–2.7 Mb, it has the most repeated DNA sequences among bacterial genomes sequenced so far. The disease, scrub typhus, occurs when infected mite larvae accidentally bite humans. Primarily indicated by undifferentiated febrile illnesses, the infection can be complicated and often fatal.

Rickettsia conorii is a Gram-negative, obligate intracellular bacterium of the genus Rickettsia that causes human disease called boutonneuse fever, Mediterranean spotted fever, Israeli tick typhus, Astrakhan spotted fever, Kenya tick typhus, Indian tick typhus, or other names that designate the locality of occurrence while having distinct clinical features. It is a member of the spotted fever group and the most geographically dispersed species in the group, recognized in most of the regions bordering on the Mediterranean Sea and Black Sea, Israel, Kenya, and other parts of North, Central, and South Africa, and India. The prevailing vector is the brown dog tick, Rhipicephalus sanguineus. The bacterium was isolated by Emile Brumpt in 1932 and named after A. Conor, who in collaboration with A. Bruch, provided the first description of boutonneuse fever in Tunisia in 1910.
Rickettsia typhi is a small, aerobic, obligate intracellular, rod shaped gram negative bacterium. It belongs to the typhus group of the Rickettsia genus, along with R. prowazekii. R. typhi has an uncertain history, as it may have long gone shadowed by epidemic typhus. This bacterium is recognized as a biocontainment level 2/3 organism. R. typhi is a flea-borne disease that is best known to be the causative agent for the disease murine typhus, which is an endemic typhus in humans that is distributed worldwide. As with all rickettsial organisms, R. typhi is a zoonotic agent that causes the disease murine typhus, displaying non-specific mild symptoms of fevers, headaches, pains and rashes. There are two cycles of R. typhi transmission from animal reservoirs containing R. typhi to humans: a classic rat-flea-rat cycle that is most well studied and common, and a secondary periodomestic cycle that could involve cats, dogs, opossums, sheep, and their fleas.

African tick bite fever (ATBF) is a bacterial infection spread by the bite of a tick. Symptoms may include fever, headache, muscle pain, and a rash. At the site of the bite there is typically a red skin sore with a dark center. The onset of symptoms usually occurs 4–10 days after the bite. Complications are rare but may include joint inflammation. Some people do not develop symptoms.
Rickettsia australis is a bacterium that causes a medical condition called Queensland tick typhus. The probable vectors are the tick species, Ixodes holocyclus and Ixodes tasmani. Small marsupials are suspected reservoirs of this bacterium.

Rickettsia parkeri is a gram-negative intracellular bacterium. The organism is found in the Western Hemisphere and is transmitted via the bite of hard ticks of the genus Amblyomma. R. parkeri causes mild spotted fever disease in humans, whose most common signs and symptoms are fever, an eschar at the site of tick attachment, rash, headache, and muscle aches. Doxycycline is the most common drug used to reduce the symptoms associated with disease.
Rickettsia felis is a species of bacterium, the pathogen that causes cat-flea typhus in humans, also known as flea-borne spotted fever. Rickettsia felis also is regarded as the causative organism of many cases of illnesses generally classed as fevers of unknown origin in humans in Africa.

Rickettsia sibirica is a species of Rickettsia. This bacterium is the etiologic agent of North Asian tick typhus, which is also known as Siberian tick typhus. The ticks that transmit it are primarily various species of Dermacentor and Haemaphysalis.
Rickettsia japonica is a species of Rickettsia. It can cause Japanese spotted fever.
Rickettsia peacockii is a species of gram negative Alphaproteobacteria of the spotted fever group, identified from Rocky Mountain wood ticks. Its type strain is SkalkahoT. The organism is passed transstadially and transovarially, and infections are localized in ovarial tissues.
"Tropheryma whipplei" is a bacterium that is the causative organism of Whipple's disease, and rarely, endocarditis.
Reductive evolution is the process by which microorganisms remove genes from their genome. It can occur when bacteria found in a free-living state enter a restrictive state or are completely absorbed by another organism becoming intracellular (symbiogenesis). The bacteria will adapt to survive and thrive in the restrictive state by altering and reducing its genome to get rid of the newly redundant pathways that are provided by the host. In an endosymbiont or symbiogenesis relationship where both the guest and host benefit, the host can also undergo reductive evolution to eliminate pathways that are more efficiently provided for by the guest.








