
Oxytetracycline is a broad-spectrum tetracycline antibiotic, the second of the group to be discovered.

Streptomyces is the largest genus of Actinomycetota, and the type genus of the family Streptomycetaceae. Over 700 species of Streptomyces bacteria have been described. As with the other Actinomycetota, streptomycetes are gram-positive, and have very large genomes with high GC content. Found predominantly in soil and decaying vegetation, most streptomycetes produce spores, and are noted for their distinct "earthy" odor that results from production of a volatile metabolite, geosmin. Different strains of the same species may colonize very diverse environments.
In organic chemistry, polyketides are a class of natural products derived from a precursor molecule consisting of a chain of alternating ketone and methylene groups: [−C(=O)−CH2−]n. First studied in the early 20th century, discovery, biosynthesis, and application of polyketides has evolved. It is a large and diverse group of secondary metabolites caused by its complex biosynthesis which resembles that of fatty acid synthesis. Because of this diversity, polyketides can have various medicinal, agricultural, and industrial applications. Many polyketides are medicinal or exhibit acute toxicity. Biotechnology has enabled discovery of more naturally-occurring polyketides and evolution of new polyketides with novel or improved bioactivity.

Platensimycin, a metabolite of Streptomyces platensis, is an antibiotic, which acts by blocking the enzymes β-ketoacyl-(acyl-carrier-protein ) synthase I/II (FabF/B).
Polyketide synthases (PKSs) are a family of multi-domain enzymes or enzyme complexes that produce polyketides, a large class of secondary metabolites, in bacteria, fungi, plants, and a few animal lineages. The biosyntheses of polyketides share striking similarities with fatty acid biosynthesis.

Isomigrastatin is an analogue of migrastatin, an organic compound that naturally occurs in the Streptomyces platensis bacteria. Isomigrastatin has shown promise as a drug in the treatment of cancer. A laboratory synthesis was reported in 2007.

Macroketones are macrocyclic compounds that contain a ketone functional group. Macroketones form the central rings systems of some synthetic polyketide antibiotics.
Streptomyces nodosus is a bacterial species in the genus Streptomyces.
Streptomyces isolates have yielded the majority of human, animal, and agricultural antibiotics, as well as a number of fundamental chemotherapy medicines. Streptomyces is the largest antibiotic-producing genus of Actinomycetota, producing chemotherapy, antibacterial, antifungal, antiparasitic drugs, and immunosuppressants. Streptomyces isolates are typically initiated with the aerial hyphal formation from the mycelium.
Streptomyces glaucescens is a bacterium species from the genus of Streptomyces which has been isolated from soil. Streptomyces glaucescens produces tetracenomycin C, tetracenomycin D and tetracenomycin E.
Streptomyces halstedii is a bacterium species from the genus of Streptomyces which has been isolated from deeper soil layers. Streptomyces halstedii produces magnamycin B, vicenistatin deltamycin A2, deltamycin A3, bafilomycin B1 and bafilomycin C1. Streptomyces halstedii also produces complex antifungal antibiotics like oligomycins and the antibiotics anisomycin and sinefungin.
Streptomyces niveus is a bacterium species from the genus of Streptomyces which has been isolated from soil in the United States. Streptomyces niveus produces the aminocoumarin antibiotic novobiocin and the compounds nivetetracyclate A and nivetetracyclate B.
Lactimidomycin is a glutarimide antibiotic derived from the bacteria Streptomyces amphibiosporus. It has antifungal, antiviral and anti-cancer properties, acting as a direct inhibitor of protein translation in ribosomes. Antiviral activity is seen against a variety of RNA viruses including flaviviruses such as dengue fever, Kunjin virus and Modoc virus, as well as vesicular stomatitis virus and poliovirus. As lactimidomycin is a natural product containing an unusual unsaturated 12-membered lactone ring, it has been the subject of numerous total synthesis approaches.
Streptomyces pulveraceus is a bacterium species from the genus of Streptomyces which has been isolated from soil in Fukuchiyama in Japan. Streptomyces pulveraceus produces zygomycine and fostriecin.
Streptomyces resistomycificus is a bacterium species from the genus of Streptomyces which has been isolated from soil. Streptomyces resistomycificus produces the pentacyclic polyketide resistomycin.
Streptomyces roseofulvus is a bacterium species from the genus of Streptomyces which has been isolated from soil. Streptomyces roseofulvus produces deoxyfrenolicin and frenolicin B.
Streptomyces spiroverticillatus is a bacterium species from the genus of Streptomyces which has been isolated from soil in Japan. Streptomyces spiroverticillatus produces tautomycin.
Lydicamycin is an organic compound with the molecular formula C47H74N4O10. Lydicamycin is an antibiotic with activity against Gram-positive bacteria. The bacteria Streptomyces lydicamycinicus and Streptomyces platensis produces lydicamycin.
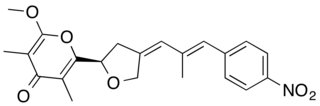
Aureothin is a natural product of a cytotoxic shikimate-polyketide antibiotic with the molecular formula C22H23NO6. Aureothin is produced by the bacterium Streptomyces thioluteus that illustrates antitumor, antifungal, and insecticidal activities and the new aureothin derivatives can be antifungal and antiproliferative. In addition, aureothin, a nitro compound from Streptomyces thioluteus, was indicated to have pesticidal activity against the bean weevil by interfering with mitochondrial respiratory complex II.
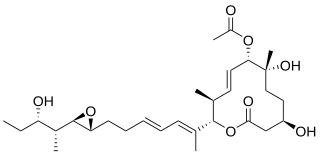
Pladienolide B is a natural product produced by bacterial strain, Streptomyces platensis MER-11107, which is a gram-positive bacteria isolated from soil in Japan. Pladienolide B is a molecule of interest due to its potential anti-cancer properties. Its anti-cancer mode of action includes binding to the SF3B complex in the U2 snRNP in the human spliceosome.






