The Wittig reaction or Wittig olefination is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide called a Wittig reagent. Wittig reactions are most commonly used to convert aldehydes and ketones to alkenes. Most often, the Wittig reaction is used to introduce a methylene group using methylenetriphenylphosphorane (Ph3P=CH2). Using this reagent, even a sterically hindered ketone such as camphor can be converted to its methylene derivative.

Organoborane or organoboron compounds are chemical compounds of boron and carbon that are organic derivatives of BH3, for example trialkyl boranes. Organoboron chemistry or organoborane chemistry is the chemistry of these compounds.

The Weinreb–Nahm ketone synthesis is a chemical reaction used in organic chemistry to make carbon–carbon bonds. It was discovered in 1981 by Steven M. Weinreb and Steven Nahm as a method to synthesize ketones. The original reaction involved two subsequent nucleophilic acyl substitutions: the conversion of an acid chloride with N,O-Dimethylhydroxylamine, to form a Weinreb–Nahm amide, and subsequent treatment of this species with an organometallic reagent such as a Grignard reagent or organolithium reagent. Nahm and Weinreb also reported the synthesis of aldehydes by reduction of the amide with an excess of lithium aluminum hydride.

The epothilones are a class of potential cancer drugs. Like taxanes, they prevent cancer cells from dividing by interfering with tubulin, but in early trials epothilones have better efficacy and milder adverse effects than taxanes.

Organozinc compounds in organic chemistry contain carbon to zinc chemical bonds. Organozinc chemistry is the science of organozinc compounds describing their physical properties, synthesis and reactions.
Polyketide synthases (PKSs) are a family of multi-domain enzymes or enzyme complexes that produce polyketides, a large class of secondary metabolites, in bacteria, fungi, plants, and a few animal lineages. The biosyntheses of polyketides share striking similarities with fatty acid biosynthesis.
In enzymology, an erythronolide synthase is an enzyme that catalyzes the chemical reaction

Hydrogen auto-transfer, also known as borrowing hydrogen, is the activation of a chemical reaction by temporary transfer of two hydrogen atoms from the reactant to a catalyst and return of those hydrogen atoms back to a reaction intermediate to form the final product. Two major classes of borrowing hydrogen reactions exist: (a) those that result in hydroxyl substitution, and (b) those that result in carbonyl addition. In the former case, alcohol dehydrogenation generates a transient carbonyl compound that is subject to condensation followed by the return of hydrogen. In the latter case, alcohol dehydrogenation is followed by reductive generation of a nucleophile, which triggers carbonyl addition. As borrowing hydrogen processes avoid manipulations otherwise required for discrete alcohol oxidation and the use of stoichiometric organometallic reagents, they typically display high levels of atom-economy and, hence, are viewed as examples of Green chemistry.

Monocerin is a dihydroisocoumarin and a polyketide metabolite that originates from various fungal species. It has been shown to display antifungal, plant pathogenic, and insecticidal characteristics. Monocerin has been isolated from Dreschlera monoceras, D. ravenelii, Exserohilum turcicum, and Fusarium larvarum.

Anthracimycin is a polyketide antibiotic discovered in 2013. Anthracimycin is derived from marine actinobacteria. In preliminary laboratory research, it has shown activity against Bacillus anthracis, the bacteria that causes anthrax, and against methicillin-resistant Staphylococcus aureus (MRSA).
In organic chemistry, carbonyl allylation describes methods for adding an allyl anion to an aldehyde or ketone to produce a homoallylic alcohol. The carbonyl allylation was first reported in 1876 by Alexander Zaitsev and employed an allylzinc reagent.
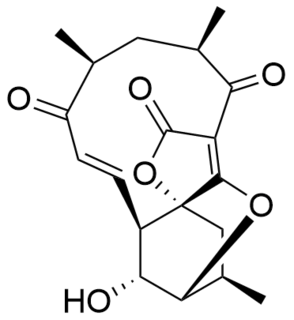
Atrop-abyssomicin C is a polycyclic polyketide-type natural product that is the atropisomer of abyssomicin C. It is a spirotetronate that belongs to the class of tetronate antibiotics, which includes compounds such as tetronomycin, agglomerin, and chlorothricin. In 2006, the Nicolaou group discovered atrop-abyssomicin C while working on the total synthesis of abyssomicin C. Then in 2007, Süssmuth and co-workers isolated atrop-abyssomicin C from Verrucosispora maris AB-18-032, a marine actinomycete found in sediment of the Japanese sea. They found that atrop-abyssomicin C was the major metabolite produced by this strain, while abyssomicin C was a minor product. The molecule displays antibacterial activity by inhibiting the enzyme PabB, thereby depleting the biosynthesis of p-aminobenzoate.

The Krische allylation involves the enantioselective iridium-catalyzed addition of an allyl group to an aldehyde or an alcohol, resulting in the formation of a secondary homoallylic alcohol. The mechanism of the Krische allylation involves primary alcohol dehydrogenation or, when using aldehyde reactants, hydrogen transfer from 2-propanol. Unlike other allylation methods, the Krische allylation avoids the use of preformed allyl metal reagents and enables the direct conversion of primary alcohols to secondary homoallylic alcohols.

Bis(cyclopentadienyl)titanium(III) chloride, also known as the Nugent–RajanBabu reagent, is the organotitanium compound which exists as a dimer with the formula [(C5H5)2TiCl]2. It is an air sensitive green solid. The complex finds specialized use in synthetic organic chemistry as a single electron reductant.

Dihydromaltophilin, or heat stable anti-fungal factor (HSAF), is a secondary metabolite of Streptomyces sp. and Lysobacter enzymogenes. HSAF is a polycyclic tetramate lactam containing a single tetramic acid unit and a 5,5,6-tricyclic system. HSAF has been shown to have anti-fungal activity mediated through the disruption of the biosynthesis of Sphingolipid's by targeting a ceramide synthase unique to fungi.
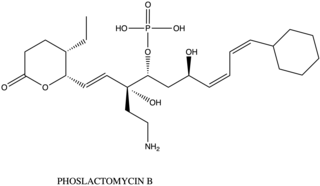
Phoslactomycin (PLM) is a natural product from the isolation of Streptomyces species. This is an inhibitor of the protein serine/threonine phosphatase which is the protein phosphate 2A (PP2A). The PP2A involves the growth factor of the cell such as to induce the formation of mitogen-activated protein interaction and playing a role in cell division and signal transduction. Therefore, PLM is used for the drug that prevents the tumor, cancer, or bacteria. There are nowsaday has 7 kinds of different PLM from PLM A to PLM G which differ the post-synthesis from the biosynthesis of PLM.
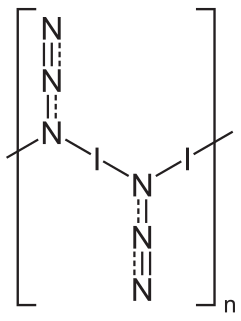
Iodine azide is an explosive inorganic compound, which in ordinary conditions is a yellow solid. Formally, it is an inter-pseudohalogen.
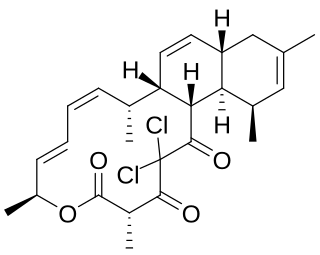
Chlorotonil A is a polyketide natural product produced by the myxobacterium Sorangium cellulosum So ce1525. It displays antimalarial activity in an animal model, and has in vitro antibacterial and antifungal activity. The activity of chlorotonil A has been attributed to the gem-dichloro-1,3-dione moiety, which is a unique functionality in polyketides. In addition to its unique halogenation, the structure of chlorotonil A has also garnered interest due to its similarity to anthracimycin, a polyketide natural product with antibiotic activity against Gram-positive bacteria.
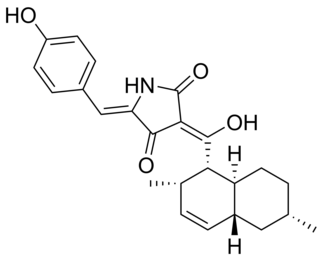
Conipyridoin E is a tetramic acid derivative produced by the fungus Coniochaeta cephalothecoides which was found on a Tibetan Plateau. This natural product has been shown to exhibit antibacterial and antifungal activity against a variety of bacteria, such as Staphylococcus aureus, methicillin-resistant Staphyloccusaureus, and Enterococcus faecalis with MIC50 values of around 0.97 μM. Isolation of a number of analogs of conipyridoin has been accomplished by Han et al. in order to discover novel antibiotic natural products to combat antibiotic resistance.
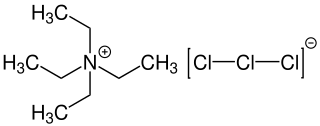
Tetraethylammonium trichloride (also known as Mioskowski reagent) is an chemical compound with the formula [NEt4][Cl3] consisting of a tetraethylammonium cation and a trichloride as anion. The trichloride is also known as trichlorine monoanion representing one of the simplest polychlorine anions. Tetraethylammonium trichloride is used as reagent for chlorinations and oxidation reactions.




















