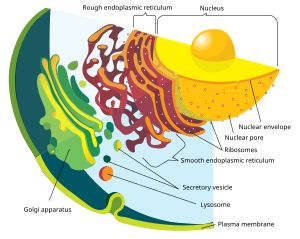
The cell is the basic structural and functional unit of all forms of life. Every cell consists of cytoplasm enclosed within a membrane; many cells contain organelles, each with a specific function. The term comes from the Latin word cellula meaning 'small room'. Most cells are only visible under a microscope. Cells emerged on Earth about 4 billion years ago. All cells are capable of replication, protein synthesis, and motility.

A microorganism, or microbe, is an organism of microscopic size, which may exist in its single-celled form or as a colony of cells.
In cell biology, an organelle is a specialized subunit, usually within a cell, that has a specific function. The name organelle comes from the idea that these structures are parts of cells, as organs are to the body, hence organelle, the suffix -elle being a diminutive. Organelles are either separately enclosed within their own lipid bilayers or are spatially distinct functional units without a surrounding lipid bilayer. Although most organelles are functional units within cells, some function units that extend outside of cells are often termed organelles, such as cilia, the flagellum and archaellum, and the trichocyst.

In biology, a kingdom is the second highest taxonomic rank, just below domain. Kingdoms are divided into smaller groups called phyla.
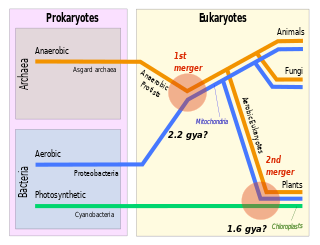
Symbiogenesis is the leading evolutionary theory of the origin of eukaryotic cells from prokaryotic organisms. The theory holds that mitochondria, plastids such as chloroplasts, and possibly other organelles of eukaryotic cells are descended from formerly free-living prokaryotes taken one inside the other in endosymbiosis. Mitochondria appear to be phylogenetically related to Rickettsiales bacteria, while chloroplasts are thought to be related to cyanobacteria.
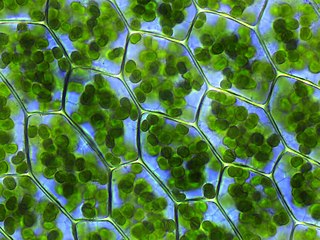
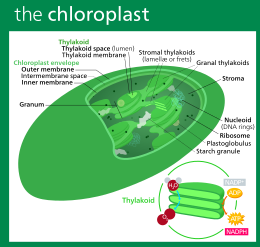
A plastid is a membrane-bound organelle found in the cells of plants, algae, and some other eukaryotic organisms. Plastids are considered to be intracellular endosymbiotic cyanobacteria.
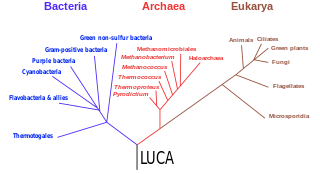
The three-domain system is a taxonomic classification system that groups all cellular life into three domains, namely Archaea, Bacteria and Eukarya, introduced by Carl Woese, Otto Kandler and Mark Wheelis in 1990. The key difference from earlier classifications such as the two-empire system and the five-kingdom classification is the splitting of Archaea from Bacteria as completely different organisms.

Chromista is a proposed but polyphyletic biological kingdom, refined from the Chromalveolata, consisting of single-celled and multicellular eukaryotic species that share similar features in their photosynthetic organelles (plastids). It includes all eukaryotes whose plastids contain chlorophyll c and are surrounded by four membranes. If the ancestor already possessed chloroplasts derived by endosymbiosis from red algae, all non-photosynthetic Chromista have secondarily lost the ability to photosynthesise. Its members might have arisen independently as separate evolutionary groups from the last eukaryotic common ancestor.

A unicellular organism, also known as a single-celled organism, is an organism that consists of a single cell, unlike a multicellular organism that consists of multiple cells. Organisms fall into two general categories: prokaryotic organisms and eukaryotic organisms. Most prokaryotes are unicellular and are classified into bacteria and archaea. Many eukaryotes are multicellular, but some are unicellular such as protozoa, unicellular algae, and unicellular fungi. Unicellular organisms are thought to be the oldest form of life, with early protocells possibly emerging 3.5–4.1 billion years ago.
A multicellular organism is an organism that consists of more than one cell, unlike unicellular organisms. All species of animals, land plants and most fungi are multicellular, as are many algae, whereas a few organisms are partially uni- and partially multicellular, like slime molds and social amoebae such as the genus Dictyostelium.

The green algae are a group of chlorophyll-containing autotrophic eukaryotes consisting of the phylum Prasinodermophyta and its unnamed sister group that contains the Chlorophyta and Charophyta/Streptophyta. The land plants (Embryophytes) have emerged deep within the charophytes as a sister of the Zygnematophyceae. Since the realization that the Embryophytes emerged within the green algae, some authors are starting to include them. The completed clade that includes both green algae and embryophytes is monophyletic and is referred to as the clade Viridiplantae and as the kingdom Plantae. The green algae include unicellular and colonial flagellates, most with two flagella per cell, as well as various colonial, coccoid (spherical), and filamentous forms, and macroscopic, multicellular seaweeds. There are about 22,000 species of green algae, many of which live most of their lives as single cells, while other species form coenobia (colonies), long filaments, or highly differentiated macroscopic seaweeds.

The Archaeplastida are a major group of eukaryotes, comprising the photoautotrophic red algae (Rhodophyta), green algae, land plants, and the minor group glaucophytes. It also includes the non-photosynthetic lineage Rhodelphidia, a predatorial (eukaryotrophic) flagellate that is sister to the Rhodophyta, and probably the microscopic picozoans. The Archaeplastida have chloroplasts that are surrounded by two membranes, suggesting that they were acquired directly through a single endosymbiosis event by phagocytosis of a cyanobacterium. All other groups which have chloroplasts, besides the amoeboid genus Paulinella, have chloroplasts surrounded by three or four membranes, suggesting they were acquired secondarily from red or green algae. Unlike red and green algae, glaucophytes have never been involved in secondary endosymbiosis events.

A prokaryote is a single-celled organism whose cell lacks a nucleus and other membrane-bound organelles. The word prokaryote comes from the Ancient Greek πρό (pró), meaning 'before', and κάρυον (káruon), meaning 'nut' or 'kernel'. In the earlier two-empire system arising from the work of Édouard Chatton, prokaryotes were classified within the empire Prokaryota. However, in the three-domain system, based upon molecular phylogenetics, prokaryotes are divided into two domains: Bacteria and Archaea. A third domain, Eukaryota, consists of organisms with nuclei.
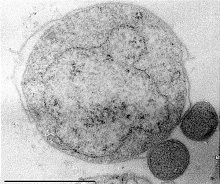
The eocyte hypothesis in evolutionary biology proposes that the eukaryotes originated from a group of prokaryotes called eocytes. After his team at the University of California, Los Angeles discovered eocytes in 1984, James A. Lake formulated the hypothesis as "eocyte tree" that proposed eukaryotes as part of archaea. Lake hypothesised the tree of life as having only two primary branches: prokaryotes, which include Bacteria and Archaea, and karyotes, that comprise Eukaryotes and eocytes. Parts of this early hypothesis were revived in a newer two-domain system of biological classification which named the primary domains as Archaea and Bacteria.
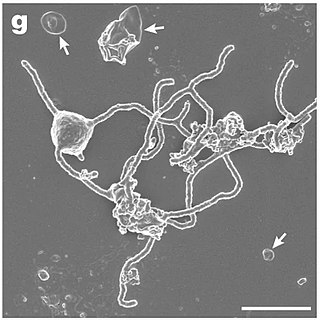
Lokiarchaeota is a proposed phylum of the Archaea. The phylum includes all members of the group previously named Deep Sea Archaeal Group, also known as Marine Benthic Group B. Lokiarchaeota is part of the superphylum Asgard containing the phyla: Lokiarchaeota, Thorarchaeota, Odinarchaeota, Heimdallarchaeota, and Helarchaeota. A phylogenetic analysis disclosed a monophyletic grouping of the Lokiarchaeota with the eukaryotes. The analysis revealed several genes with cell membrane-related functions. The presence of such genes support the hypothesis of an archaeal host for the emergence of the eukaryotes; the eocyte-like scenarios.
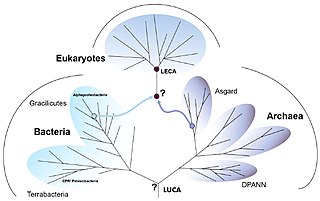
Eukaryogenesis, the process which created the eukaryotic cell and lineage, is a milestone in the evolution of life, since eukaryotes include all complex cells and almost all multicellular organisms. The process is widely agreed to have involved symbiogenesis, in which an archeon and a bacterium came together to create the first eukaryotic common ancestor (FECA). This cell had a new level of complexity and capability, with a nucleus, at least one centriole and cilium, facultatively aerobic mitochondria, sex, a dormant cyst with a cell wall of chitin and/or cellulose and peroxisomes. It evolved into a population of single-celled organisms that included the last eukaryotic common ancestor (LECA), gaining capabilities along the way, though the sequence of the steps involved has been disputed, and may not have started with symbiogenesis. In turn, the LECA gave rise to the eukaryotes' crown group, containing the ancestors of animals, fungi, plants, and a diverse range of single-celled organisms.

A plastid is a membrane-bound organelle found in plants, algae and other eukaryotic organisms that contribute to the production of pigment molecules. Most plastids are photosynthetic, thus leading to color production and energy storage or production. There are many types of plastids in plants alone, but all plastids can be separated based on the number of times they have undergone endosymbiotic events. Currently there are three types of plastids; primary, secondary and tertiary. Endosymbiosis is reputed to have led to the evolution of eukaryotic organisms today, although the timeline is highly debated.

Marine prokaryotes are marine bacteria and marine archaea. They are defined by their habitat as prokaryotes that live in marine environments, that is, in the saltwater of seas or oceans or the brackish water of coastal estuaries. All cellular life forms can be divided into prokaryotes and eukaryotes. Eukaryotes are organisms whose cells have a nucleus enclosed within membranes, whereas prokaryotes are the organisms that do not have a nucleus enclosed within a membrane. The three-domain system of classifying life adds another division: the prokaryotes are divided into two domains of life, the microscopic bacteria and the microscopic archaea, while everything else, the eukaryotes, become the third domain.

Marine protists are defined by their habitat as protists that live in marine environments, that is, in the saltwater of seas or oceans or the brackish water of coastal estuaries. Life originated as marine single-celled prokaryotes and later evolved into more complex eukaryotes. Eukaryotes are the more developed life forms known as plants, animals, fungi and protists. Protists are the eukaryotes that cannot be classified as plants, fungi or animals. They are mostly single-celled and microscopic. The term protist came into use historically as a term of convenience for eukaryotes that cannot be strictly classified as plants, animals or fungi. They are not a part of modern cladistics because they are paraphyletic.
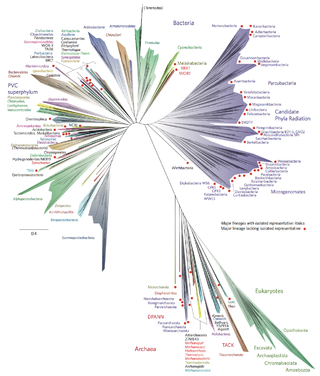
The two-domain system is a biological classification by which all organisms in the tree of life are classified into two domains, Bacteria and Archaea. It emerged from development of knowledge of archaea diversity and challenges the widely accepted three-domain system that classifies life into Bacteria, Archaea, and Eukarya. It was preceded by the eocyte hypothesis of James A. Lake in the 1980s, which was largely superseded by the three-domain system, due to evidence at the time. Better understanding of archaea, especially of their roles in the origin of eukaryotes through symbiogenesis with bacteria, led to the revival of the eocyte hypothesis in the 2000s. The two-domain system became more widely accepted after the discovery of a large group (superphylum) of Archaea called Asgard in 2017, which evidence suggests to be the evolutionary root of eukaryotes, thereby making eukaryotes members of the domain Archaea.