| Nuclear envelope | |
|---|---|
 Human cell nucleus | |
| Identifiers | |
| TH | H1.00.01.2.01001 |
| FMA | 63888 |
| Anatomical terminology | |
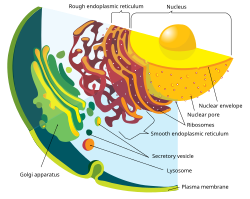
The nuclear envelope, also known as the nuclear membrane, [1] [a] is made up of two lipid bilayer membranes that in eukaryotic cells surround the nucleus, which encloses the genetic material.
Contents
- Structure
- Outer membrane
- Inner membrane
- Nuclear pores
- Cell division
- Breakdown
- Reformation
- Origin of the nuclear membrane
- Notes
- References
- External links
The nuclear envelope consists of two lipid bilayer membranes: an inner nuclear membrane and an outer nuclear membrane. [4] The space between the membranes is called the perinuclear space. It is usually about 10–50 nm wide. [5] [6] The outer nuclear membrane is continuous with the endoplasmic reticulum membrane. [4] The nuclear envelope has many nuclear pores that allow materials to move between the cytosol and the nucleus. [4] Intermediate filament proteins called lamins form a structure called the nuclear lamina on the inner aspect of the inner nuclear membrane and give structural support to the nucleus. [4]