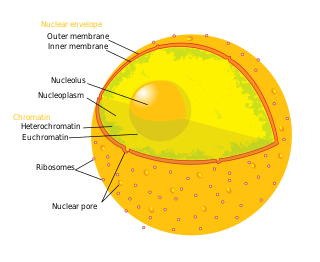
The cell nucleus is a membrane-bound organelle found in eukaryotic cells. Eukaryotic cells usually have a single nucleus, but a few cell types, such as mammalian red blood cells, have no nuclei, and a few others including osteoclasts have many. The main structures making up the nucleus are the nuclear envelope, a double membrane that encloses the entire organelle and isolates its contents from the cellular cytoplasm; and the nuclear matrix, a network within the nucleus that adds mechanical support.
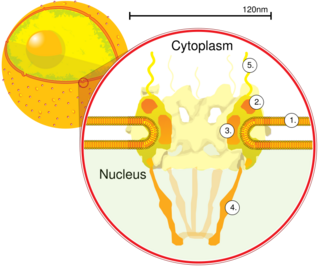
The nuclear pore complex (NPC), is a large protein complex giving rise to the nuclear pore. Nuclear pores are found in the nuclear envelope that surrounds the cell nucleus in eukaryotic cells. The nuclear envelope is studded by a great number of nuclear pores that give access to various molecules, to and from the nucleoplasm and the cytoplasm. Small molecules can diffuse easily but other larger molecules need to be transported across.

The nucleoplasm, also known as karyoplasm, is the type of protoplasm that makes up the cell nucleus, the most prominent organelle of the eukaryotic cell. It is enclosed by the nuclear envelope, also known as the nuclear membrane. The nucleoplasm resembles the cytoplasm of a eukaryotic cell in that it is a gel-like substance found within a membrane, although the nucleoplasm only fills out the space in the nucleus and has its own unique functions. The nucleoplasm suspends structures within the nucleus that are not membrane-bound and is responsible for maintaining the shape of the nucleus. The structures suspended in the nucleoplasm include chromosomes, various proteins, nuclear bodies, the nucleolus, nucleoporins, nucleotides, and nuclear speckles.
A nuclear localization signalorsequence (NLS) is an amino acid sequence that 'tags' a protein for import into the cell nucleus by nuclear transport. Typically, this signal consists of one or more short sequences of positively charged lysines or arginines exposed on the protein surface. Different nuclear localized proteins may share the same NLS. An NLS has the opposite function of a nuclear export signal (NES), which targets proteins out of the nucleus.
Karyopherins are proteins involved in transporting molecules between the cytoplasm and the nucleus of a eukaryotic cell. The inside of the nucleus is called the karyoplasm. Generally, karyopherin-mediated transport occurs through nuclear pores which act as a gateway into and out of the nucleus. Most proteins require karyopherins to traverse the nuclear pore.
Importin is a type of karyopherin that transports protein molecules from the cell's cytoplasm to the nucleus. It does so by binding to specific recognition sequences, called nuclear localization sequences (NLS).

Ran also known as GTP-binding nuclear protein Ran is a protein that in humans is encoded by the RAN gene. Ran is a small 25 kDa protein that is involved in transport into and out of the cell nucleus during interphase and also involved in mitosis. It is a member of the Ras superfamily.
Nuclear transport refers to the mechanisms by which molecules move across the nuclear membrane of a cell. The entry and exit of large molecules from the cell nucleus is tightly controlled by the nuclear pore complexes (NPCs). Although small molecules can enter the nucleus without regulation, macromolecules such as RNA and proteins require association with transport factors known as nuclear transport receptors, like karyopherins called importins to enter the nucleus and exportins to exit.
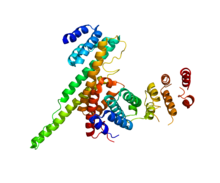
Nuclear pore glycoprotein p62 is a protein complex associated with the nuclear envelope. The p62 protein remains associated with the nuclear pore complex-lamina fraction. p62 is synthesized as a soluble cytoplasmic precursor of 61 kDa followed by modification that involve addition of N-acetylglucosamine residues, followed by association with other complex proteins. In humans it is encoded by the NUP62 gene.

The nuclear envelope, also known as the nuclear membrane, is made up of two lipid bilayer membranes that in eukaryotic cells surround the nucleus, which encloses the genetic material.

Importin subunit beta-1 is a protein that in humans is encoded by the KPNB1 gene.
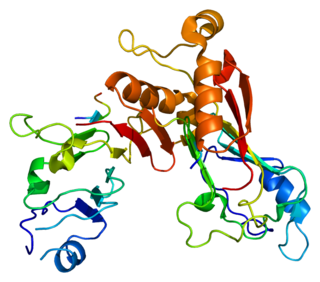
Nuclear pore complex protein Nup98-Nup96 is a protein that in humans is encoded by the NUP98 gene.

Transportin-1 is a protein that in humans is encoded by the TNPO1 gene.

Nucleoporin 214 (Nup2014) is a protein that in humans is encoded by the NUP214 gene.

Nucleoporin 88 (Nup88) is a protein that in humans is encoded by the NUP88 gene.

Nucleoporin 54 (Nup54) is a protein that in humans is encoded by the NUP54 gene.

Nuclear pore complex protein Nup133, or Nucleoporin Nup133, is a protein that in humans is encoded by the NUP133 gene.

Nuclear envelope pore membrane protein POM 121 is a protein that in humans is encoded by the POM121 gene. Alternatively spliced variants that encode different protein isoforms have been described but the full-length nature of only one has been determined.

Nucleoporin 43 (Nup43) is a protein that in humans is encoded by the NUP43 gene.
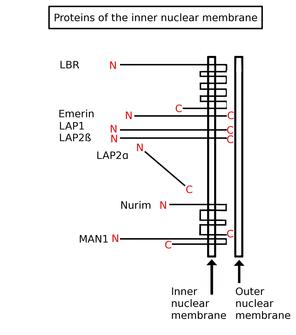
Inner nuclear membrane proteins are membrane proteins that are embedded in or associated with the inner membrane of the nuclear envelope. There are about 60 INM proteins, most of which are poorly characterized with respect to structure and function. Among the few well-characterized INM proteins are lamin B receptor (LBR), lamina-associated polypeptide 1 (LAP1), lamina-associated polypeptide-2 (LAP2), emerin and MAN1.