
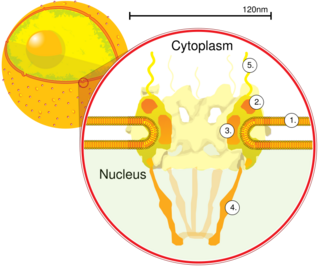
A nuclear pore is a part of a large complex of proteins, known as a nuclear pore complex that spans the nuclear envelope, which is the double membrane surrounding the eukaryotic cell nucleus. There are approximately 1,000 nuclear pore complexes (NPCs) in the nuclear envelope of a vertebrate cell, but it varies depending on cell type and the stage in the life cycle. The human nuclear pore complex (hNPC) is a 110 megadalton (MDa) structure. The proteins that make up the nuclear pore complex are known as nucleoporins; each NPC contains at least 456 individual protein molecules and is composed of 34 distinct nucleoporin proteins.About half of the nucleoporins typically contain solenoid protein domains—either an alpha solenoid or a beta-propeller fold, or in some cases both as separate structural domains. The other half show structural characteristics typical of "natively unfolded" or intrinsically disordered proteins, i.e. they are highly flexible proteins that lack ordered tertiary structure. These disordered proteins are the FG nucleoporins, so called because their amino-acid sequence contains many phenylalanine—glycine repeats.

Ran also known as GTP-binding nuclear protein Ran is a protein that in humans is encoded by the RAN gene. Ran is a small 25 kDa protein that is involved in transport into and out of the cell nucleus during interphase and also involved in mitosis. It is a member of the Ras superfamily.
Anti-glycoprotein-210 antibodies are directed at gp210 and are found within primary biliary cirrhosis (PBC) patients in high frequency. AGPA recognize the cytoplasmic-oriented carboxyl terminus (tail) of the protein. While AGPA is found as a prognostic marker in only a minority of PBC patients, those that did had higher mortality and were predicted a poor outcome. In addition, patients that responded to ursodeoxycholic acid (UDCA) therapy and, therefore, had AGPA reductions failed to develop end-stage liver disease relative to untreated cohort with anti-gp210 Ab. PBC patients with potentially destructive AGPA have increased expression of Nup210 in the bile duct, a potential immune tolerance-escaping factor.
Nucleoporin p62 (p62) is a protein complex associated with the nuclear envelope. The p62 protein remains associated with the nuclear pore complex-lamina fraction. p62 is synthesized as a soluble cytoplasmic precursor of 61 kDa followed by modification that involve addition of N-acetylglucosamine residues, followed by association with other complex proteins.

Nucleoporins are a family of proteins which are the constituent building blocks of the nuclear pore complex (NPC). The nuclear pore complex is a massive structure embedded in the nuclear envelope at sites where the inner and outer nuclear membranes fuse, forming a gateway that regulates the flow of macromolecules between the cell nucleus and the cytoplasm. Nuclear pores enable the passive and facilitated transport of molecules across the nuclear envelope. Nucleoporins, a family of around 30 proteins, are the main components of the nuclear pore complex in eukaryotic cells. Nucleoporin 62 is the most abundant member of this family. Nucleoporins are able to transport molecules across the nuclear envelope at a very high rate. A single NPC is able to transport 60,000 protein molecules across the nuclear envelope every minute.

Importin subunit beta-1 is a protein that in humans is encoded by the KPNB1 gene.
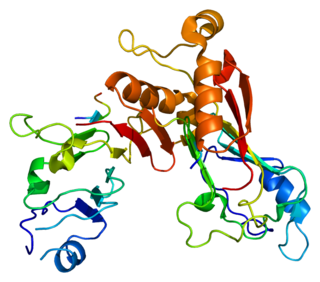
Nuclear pore complex protein Nup98-Nup96 is a protein that in humans is encoded by the NUP98 gene.

Nucleoporin 153 (Nup153) is a protein which in humans is encoded by the NUP153 gene. It is an essential component of the basket of nuclear pore complexes (NPCs) in vertebrates, and required for the anchoring of NPCs. It also acts as the docking site of an importing karyopherin. On the cytoplasmic side of the NPC, Nup358 fulfills an analogous role.

Nucleoporin 214 (Nup2014) is a protein that in humans is encoded by the NUP214 gene.

Nucleoporin 88 (Nup88) is a protein that in humans is encoded by the NUP88 gene.

Nucleoporin 50 (Nup50) is a protein that in humans is encoded by the NUP50 gene.

Nucleoporin 107 (Nup107) is a protein that in humans is encoded by the NUP107 gene.

Nucleoporin 54 (Nup54) is a protein that in humans is encoded by the NUP54 gene.

Nucleoporin 133 (Nup133) is a protein that in humans is encoded by the NUP133 gene.

Nuclear envelope pore membrane protein POM 121 is a protein that in humans is encoded by the POM121 gene. Alternatively spliced variants that encode different protein isoforms have been described but the full-length nature of only one has been determined.

Nucleoporin 160 (Nup160) is a protein that in humans is encoded by the NUP160 gene.

Nucleoporin NDC1 is a protein that in humans is encoded by the TMEM48 gene. It anchors aladin to the nuclear pore complex.

Nucleoporin 43 (Nup43) is a protein that in humans is encoded by the NUP43 gene.
Nucleoporin 37 (Nup37) is a protein that in humans is encoded by the NUP37 gene.
Gene gating is a phenomenon by which transcriptionally active genes are brought next to nuclear pore complexes (NPCs) so that nascent transcripts can quickly form mature mRNA associated with export factors. Gene gating was first hypothesised by Günter Blobel in 1985. It has been shown to occur in Saccharomyces cerevisiae, Caenorhabditis elegans, Drosophila melanogaster as well as mammalian model systems.



















