Eosinophils, sometimes called eosinophiles or, less commonly, acidophils, are a variety of white blood cells and one of the immune system components responsible for combating multicellular parasites and certain infections in vertebrates. Along with mast cells and basophils, they also control mechanisms associated with allergy and asthma. They are granulocytes that develop during hematopoiesis in the bone marrow before migrating into blood, after which they are terminally differentiated and do not multiply.

Keratinocytes are the primary type of cell found in the epidermis, the outermost layer of the skin. In humans, they constitute 90% of epidermal skin cells. Basal cells in the basal layer of the skin are sometimes referred to as basal keratinocytes. Keratinocytes form a barrier against environmental damage by heat, UV radiation, water loss, pathogenic bacteria, fungi, parasites, and viruses. A number of structural proteins, enzymes, lipids, and antimicrobial peptides contribute to maintain the important barrier function of the skin. Keratinocytes differentiate from epidermal stem cells in the lower part of the epidermis and migrate towards the surface, finally becoming corneocytes and eventually being shed, which happens every 40 to 56 days in humans.
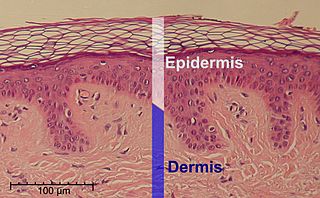
The epidermis is the outermost of the three layers that comprise the skin, the inner layers being the dermis and hypodermis. The epidermis layer provides a barrier to infection from environmental pathogens and regulates the amount of water released from the body into the atmosphere through transepidermal water loss.

Keratin 16 is a protein that in humans is encoded by the KRT16 gene.

Atopy is the tendency to produce an exaggerated immunoglobulin E (IgE) immune response to otherwise harmless substances in the environment. Allergic diseases are clinical manifestations of such inappropriate, atopic responses.

Atopic dermatitis (AD), also known as atopic eczema, is a long-term type of inflammation of the skin. AD is also often called simply eczema but the same term is also used to refer to dermatitis, the larger group of skin conditions. AD results in itchy, red, swollen, and cracked skin. Clear fluid may come from the affected areas, which can thicken over time.

Urocanic acid is an intermediate in the catabolism of L-histidine. The cis-urocanic acid isomer is rare.
Interleukin 33 (IL-33) is a protein that in humans is encoded by the IL33 gene.

Histidine ammonia-lyase is an enzyme that in humans is encoded by the HAL gene. It converts histidine into ammonia and urocanic acid. Its systematic name is L-histidine ammonia-lyase (urocanate-forming).
Corneocytes are terminally differentiated keratinocytes and compose most of the stratum corneum, the outermost layer of the epidermis. They are regularly replaced through desquamation and renewal from lower epidermal layers and are essential for its function as a skin barrier.

The neuropeptide S receptor (NPSR) is a member of the G-protein coupled receptor superfamily of integral membrane proteins which binds neuropeptide S (NPS). It was formerly an orphan receptor, GPR154, until the discovery of neuropeptide S as the endogenous ligand. Increased expression of this gene in ciliated cells of the respiratory epithelium and in bronchial smooth muscle cells is associated with asthma. This gene is a member of the G protein-coupled receptor 1 family and encodes a plasma membrane protein. Mutations in this gene have also been associated with this disease.
Keratohyalin is a protein structure found in cytoplasmic granules of the keratinocytes in the stratum granulosum of the epidermis. Keratohyalin granules (KHG) mainly consist of keratin, profilaggrin, loricrin and trichohyalin proteins which contribute to cornification or keratinization, the process of the formation of epidermal cornified cell envelope. During the keratinocyte differentiation, these granules maturate and expand in size, which leads to the conversion of keratin tonofilaments into a homogenous keratin matrix, an important step in cornification.
Kallikrein-related peptidase 7 (KLK7) is a serine protease that in humans is encoded by the KLK7 gene. KLK7 was initially purified from the epidermis and characterised as stratum corneum chymotryptic enzyme (SCCE). It was later identified as the seventh member of the human kallikrein family, which includes fifteen homologous serine proteases located on chromosome 19 (19q13).
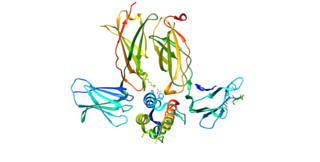
Thymic stromal lymphopoietin (TSLP) is an interleukin (IL)-2-like cytokine, alarmin, and growth factor involved in numerous physiological and pathological processes, primarily those of the immune system. It shares a common ancestor with IL-7.

Peeling skin syndrome is an autosomal recessive disorder characterized by lifelong peeling of the stratum corneum, and may be associated with pruritus, short stature, and easily removed anagen hair.
TGM5 is a transglutaminase enzyme.

William Henry Irwin McLean is an Irish geneticist who is emeritus professor of genetic medicine, at the School of Life Sciences, University of Dundee.
Mast cell activation syndrome (MCAS) is a term referring to one of two types of mast cell activation disorder (MCAD); the other type is idiopathic MCAD. MCAS is an immunological condition in which mast cells, a type of white blood cell, inappropriately and excessively release chemical mediators, such as histamine, resulting in a range of chronic symptoms, sometimes including anaphylaxis or near-anaphylaxis attacks. Primary symptoms include cardiovascular, dermatological, gastrointestinal, neurological, and respiratory problems.
The epidermal differentiation complex (EDC) is a gene complex comprising over fifty genes encoding proteins involved in the terminal differentiation and cornification of keratinocytes, the primary cell type of the epidermis. In humans, the complex is located on a 1.9 Mbp stretch within chromosome 1q21. The proteins encoded by EDC genes are closely related in terms of function, and evolutionarily they belong to three distinct gene families: the cornified envelope precursor family, the S100 protein family and the S100 fused type protein (SFTP) family.

ORMDL sphingolipid biosynthesis regulator 3 is a protein that in humans is encoded by the ORMDL3 gene. Variants affecting the expression of this gene are associated with asthma in childhood. Transgenic mice which overexpress human ORMDL3 have increased levels of IgE. This correlated with increased numbers of macrophages, neutrophils, eosinophils, CD4+ and enhanced Th2 cytokine levels in the lung tissue.