Integrins are transmembrane receptors that help cell-cell and cell-extracellular matrix (ECM) adhesion. Upon ligand binding, integrins activate signal transduction pathways that mediate cellular signals such as regulation of the cell cycle, organization of the intracellular cytoskeleton, and movement of new receptors to the cell membrane. The presence of integrins allows rapid and flexible responses to events at the cell surface.

In mammalian cells, vinculin is a membrane-cytoskeletal protein in focal adhesion plaques that is involved in linkage of integrin adhesion molecules to the actin cytoskeleton. Vinculin is a cytoskeletal protein associated with cell-cell and cell-matrix junctions, where it is thought to function as one of several interacting proteins involved in anchoring F-actin to the membrane.

In cell biology, focal adhesions are large macromolecular assemblies through which mechanical force and regulatory signals are transmitted between the extracellular matrix (ECM) and an interacting cell. More precisely, focal adhesions are the sub-cellular structures that mediate the regulatory effects of a cell in response to ECM adhesion.
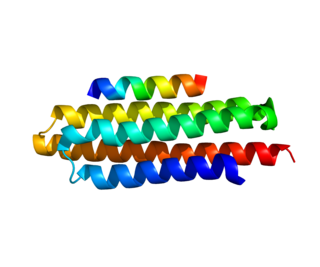
Paxillin is a protein that in humans is encoded by the PXN gene. Paxillin is expressed at focal adhesions of non-striated cells and at costameres of striated muscle cells, and it functions to adhere cells to the extracellular matrix. Mutations in PXN as well as abnormal expression of paxillin protein has been implicated in the progression of various cancers.
Actinin is a microfilament protein. The functional protein is an anti-parallel dimer, which cross-links the thin filaments in adjacent sarcomeres, and therefore coordinates contractions between sarcomeres in the horizontal axis. Alpha-actinin is a part of the spectrin superfamily. This superfamily is made of spectrin, dystrophin, and their homologous and isoforms. In non-muscle cells, it is found by the actin filaments and at the adhesion sites.The lattice like arrangement provides stability to the muscle contractile apparatus. Specifically, it helps bind actin filaments to the cell membrane. There is a binding site at each end of the rod and with bundles of actin filaments.
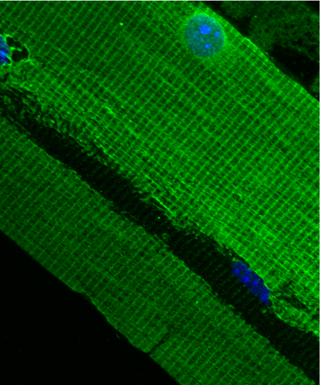
The costamere is a structural-functional component of striated muscle cells which connects the sarcomere of the muscle to the cell membrane.

Integrin-linked kinase is an enzyme that in humans is encoded by the ILK gene involved with integrin-mediated signal transduction. Mutations in ILK are associated with cardiomyopathies. It is a 59kDa protein originally identified in a yeast-two hybrid screen with integrin β1 as the bait protein. Since its discovery, ILK has been associated with multiple cellular functions including cell migration, proliferation, and adhesion.

Integrin beta-1 (ITGB1), also known as CD29, is a cell surface receptor that in humans is encoded by the ITGB1 gene. This integrin associates with integrin alpha 1 and integrin alpha 2 to form integrin complexes which function as collagen receptors. It also forms dimers with integrin alpha 3 to form integrin receptors for netrin 1 and reelin. These and other integrin beta 1 complexes have been historically known as very late activation (VLA) antigens.
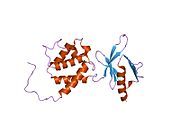
Talin is a high-molecular-weight cytoskeletal protein concentrated at regions of cell–substratum contact and, in lymphocytes, at cell–cell contacts. Discovered in 1983 by Keith Burridge and colleagues, talin is a ubiquitous cytosolic protein that is found in high concentrations in focal adhesions. It is capable of linking integrins to the actin cytoskeleton either directly or indirectly by interacting with vinculin and α-actinin.

PTK2 protein tyrosine kinase 2 (PTK2), also known as focal adhesion kinase (FAK), is a protein that, in humans, is encoded by the PTK2 gene. PTK2 is a focal adhesion-associated protein kinase involved in cellular adhesion and spreading processes. It has been shown that when FAK was blocked, breast cancer cells became less metastatic due to decreased mobility.

Transforming growth factor beta-1-induced transcript 1 protein is a protein that in humans is encoded by the TGFB1I1 gene. Often put together with and studied alongside TGFB1I1 is the mouse homologue HIC-5. As the name suggests, TGFB1I1 is an induced form of the larger family of TGFB1. Studies suggest TGFB1I1 plays a role in processes of cell growth, proliferation, migration, differentiation and senescence. TGFB1I1 is most localized at focal adhesion complexes of cells, although it may be found active in the cytosol, nucleus and cell membrane as well.

Actin, cytoplasmic 2, or gamma-actin is a protein that in humans is encoded by the ACTG1 gene. Gamma-actin is widely expressed in cellular cytoskeletons of many tissues; in adult striated muscle cells, gamma-actin is localized to Z-discs and costamere structures, which are responsible for force transduction and transmission in muscle cells. Mutations in ACTG1 have been associated with nonsyndromic hearing loss and Baraitser-Winter syndrome, as well as susceptibility of adolescent patients to vincristine toxicity.

Alpha-actinin-1 is a protein that in humans is encoded by the ACTN1 gene.

Alpha-actinin-3, also known as alpha-actinin skeletal muscle isoform 3 or F-actin cross-linking protein, is a protein that in humans is encoded by the ACTN3 gene located on chromosome 11. All people have two copies (alleles) of this gene.
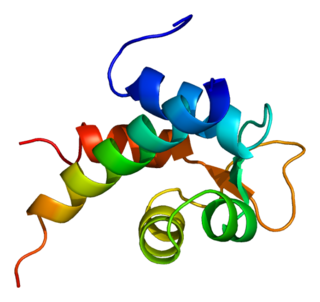
Alpha-actinin-2 is a protein which in humans is encoded by the ACTN2 gene. This gene encodes an alpha-actinin isoform that is expressed in both skeletal and cardiac muscles and functions to anchor myofibrillar actin thin filaments and titin to Z-discs.

Alpha-actinin-4 is a protein that in humans is encoded by the ACTN4 gene.
Filamin-C (FLN-C) also known as actin-binding-like protein (ABPL) or filamin-2 (FLN2) is a protein that in humans is encoded by the FLNC gene. Filamin-C is mainly expressed in cardiac and skeletal muscles, and functions at Z-discs and in subsarcolemmal regions.

Alpha-7 integrin is a protein that in humans is encoded by the ITGA7 gene. Alpha-7 integrin is critical for modulating cell-matrix interactions. Alpha-7 integrin is highly expressed in cardiac muscle, skeletal muscle and smooth muscle cells, and localizes to Z-disc and costamere structures. Mutations in ITGA7 have been associated with congenital myopathies and noncompaction cardiomyopathy, and altered expression levels of alpha-7 integrin have been identified in various forms of muscular dystrophy.

αE-catenin, also known as Catenin alpha-1 is a protein that in humans is encoded by the CTNNA1 gene. αE-catenin is highly expressed in cardiac muscle and localizes to adherens junctions at intercalated disc structures where it functions to mediate the anchorage of actin filaments to the sarcolemma. αE-catenin also plays a role in tumor metastasis and skin cell function.

Talin 2 is a protein in humans that is encoded by the TLN2 gene. It belongs to the talin protein family. This gene encodes a protein related to talin 1, a cytoskeletal protein that plays a significant role in the assembly of actin filaments. Talin-2 is expressed at high levels in cardiac muscle and functions to provide linkages between the extracellular matrix and actin cytoskeleton at costamere structures to transduce force laterally.