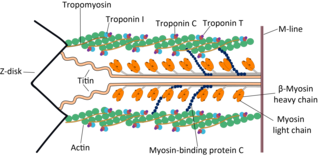
Skeletal muscle is one of the three types of vertebrate muscle tissue, the other being cardiac muscle and smooth muscle. They are part of the voluntary muscular system and typically are attached by tendons to bones of a skeleton. The skeletal muscle cells are much longer than in the other types of muscle tissue, and are also known as muscle fibers. The tissue of a skeletal muscle is striated – having a striped appearance due to the arrangement of the sarcomeres.
Troponin, or the troponin complex, is a complex of three regulatory proteins that are integral to muscle contraction in skeletal muscle and cardiac muscle, but not smooth muscle. Measurements of cardiac-specific troponins I and T are extensively used as diagnostic and prognostic indicators in the management of myocarditis, myocardial infarction and acute coronary syndrome. Blood troponin levels may be used as a diagnostic marker for stroke or other myocardial injury that is ongoing, although the sensitivity of this measurement is low.

Troponin I is a cardiac and skeletal muscle protein family. It is a part of the troponin protein complex, where it binds to actin in thin myofilaments to hold the actin-tropomyosin complex in place. Troponin I prevents myosin from binding to actin in relaxed muscle. When calcium binds to the troponin C, it causes conformational changes which lead to dislocation of troponin I. Afterwards, tropomyosin leaves the binding site for myosin on actin leading to contraction of muscle. The letter I is given due to its inhibitory character. It is a useful marker in the laboratory diagnosis of heart attack. It occurs in different plasma concentration but the same circumstances as troponin T - either test can be performed for confirmation of cardiac muscle damage and laboratories usually offer one test or the other.

Troponin I, cardiac muscle is a protein that in humans is encoded by the TNNI3 gene. It is a tissue-specific subtype of troponin I, which in turn is a part of the troponin complex.
Cardiac muscle troponin T (cTnT) is a protein that in humans is encoded by the TNNT2 gene. Cardiac TnT is the tropomyosin-binding subunit of the troponin complex, which is located on the thin filament of striated muscles and regulates muscle contraction in response to alterations in intracellular calcium ion concentration.

Tropomyosin alpha-1 chain is a protein that in humans is encoded by the TPM1 gene. This gene is a member of the tropomyosin (Tm) family of highly conserved, widely distributed actin-binding proteins involved in the contractile system of striated and smooth muscles and the cytoskeleton of non-muscle cells.

Alpha-actinin-3, also known as alpha-actinin skeletal muscle isoform 3 or F-actin cross-linking protein, is a protein that in humans is encoded by the ACTN3 gene located on chromosome 11. All people have two copies (alleles) of this gene.

Troponin C, also known as TN-C or TnC, is a protein that resides in the troponin complex on actin thin filaments of striated muscle and is responsible for binding calcium to activate muscle contraction. Troponin C is encoded by the TNNC1 gene in humans for both cardiac and slow skeletal muscle.

β-Tropomyosin, also known as tropomyosin beta chain is a protein that in humans is encoded by the TPM2 gene. β-tropomyosin is striated muscle-specific coiled coil dimer that functions to stabilize actin filaments and regulate muscle contraction.

Troponin I, slow skeletal muscle is a protein that in humans is encoded by the TNNI1 gene. It is a tissue-specific subtype of troponin I, which in turn is a part of the troponin complex.

Myosin regulatory light chain 2, ventricular/cardiac muscle isoform (MLC-2) also known as the regulatory light chain of myosin (RLC) is a protein that in humans is encoded by the MYL2 gene. This cardiac ventricular RLC isoform is distinct from that expressed in skeletal muscle (MYLPF), smooth muscle (MYL12B) and cardiac atrial muscle (MYL7).

Slow skeletal muscle troponin T (sTnT) is a protein that in humans is encoded by the TNNT1 gene.

Myosin essential light chain (ELC), ventricular/cardiac isoform is a protein that in humans is encoded by the MYL3 gene. This cardiac ventricular/slow skeletal ELC isoform is distinct from that expressed in fast skeletal muscle (MYL1) and cardiac atrial muscle (MYL4). Ventricular ELC is part of the myosin molecule and is important in modulating cardiac muscle contraction.

Atrial Light Chain-1 (ALC-1), also known as Essential Light Chain, Atrial is a protein that in humans is encoded by the MYL4 gene. ALC-1 is expressed in fetal cardiac ventricular and fetal skeletal muscle, as well as fetal and adult cardiac atrial tissue. ALC-1 expression is reactivated in human ventricular myocardium in various cardiac muscle diseases, including hypertrophic cardiomyopathy, dilated cardiomyopathy, ischemic cardiomyopathy and congenital heart diseases.

Fast skeletal muscle troponin T (fTnT) is a protein that in humans is encoded by the TNNT3 gene.

Myosin-1, also known as 'striated muscle myosin heavy chain 1', is a protein that in humans is encoded by the MYH1 gene. This gene is most highly expressed in fast type IIX/D muscle fibres of vertebrates and encodes a protein found uniquely in striated muscle; it is a class II myosin with a long coiled coil tail that dimerizes and should not be confused with 'Myosin 1' encoded by the MYO1 family of genes (MYO1A-MYO1H). Class I MYO1 genes function in many cell types throughout biology and are single-headed membrane-binding myosins that lack a long coiled coil tail.

Troponin C, skeletal muscle is a protein that in humans is encoded by the TNNC2 gene.

Myosin-3 is a protein that in humans is encoded by the MYH3 gene.

Atrial Light Chain-2 (ALC-2) also known as Myosin regulatory light chain 2, atrial isoform (MLC2a) is a protein that in humans is encoded by the MYL7 gene. ALC-2 expression is restricted to cardiac muscle atria in healthy individuals, where it functions to modulate cardiac development and contractility. In human diseases, including hypertrophic cardiomyopathy, dilated cardiomyopathy, ischemic cardiomyopathy and others, ALC-2 expression is altered.

Myosin binding protein C, fast type is a protein that in humans is encoded by the MYBPC2 gene.