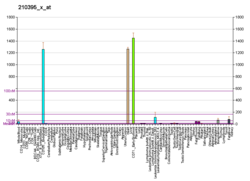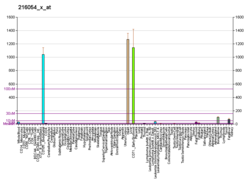
Troponin, or the troponin complex, is a complex of three regulatory proteins that are integral to muscle contraction in skeletal muscle and cardiac muscle, but not smooth muscle. Measurements of cardiac-specific troponins I and T are extensively used as diagnostic and prognostic indicators in the management of myocardial infarction and acute coronary syndrome. Blood troponin levels may be used as a diagnostic marker for stroke or other myocardial injury that is ongoing, although the sensitivity of this measurement is low.
MYH7 is a gene encoding a myosin heavy chain beta (MHC-β) isoform expressed primarily in the heart, but also in skeletal muscles. This isoform is distinct from the fast isoform of cardiac myosin heavy chain, MYH6, referred to as MHC-α. MHC-β is the major protein comprising the thick filament that forms the sarcomeres in cardiac muscle and plays a major role in cardiac muscle contraction.

Myosin regulatory light polypeptide 9 is a protein that in humans is encoded by the MYL9 gene.
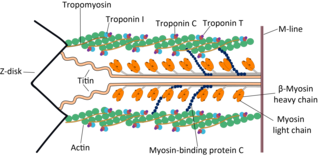
Cardiac muscle troponin T (cTnT) is a protein that in humans is encoded by the TNNT2 gene. Cardiac TnT is the tropomyosin-binding subunit of the troponin complex, which is located on the thin filament of striated muscles and regulates muscle contraction in response to alterations in intracellular calcium ion concentration.

Tropomyosin alpha-1 chain is a protein that in humans is encoded by the TPM1 gene. This gene is a member of the tropomyosin (Tm) family of highly conserved, widely distributed actin-binding proteins involved in the contractile system of striated and smooth muscles and the cytoskeleton of non-muscle cells.
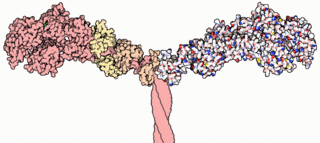
A myosin light chain is a light chain of myosin. Myosin light chains were discovered by Chinese biochemist Cao Tianqin when he was a graduate student at the University of Cambridge in England.

ACTC1 encodes cardiac muscle alpha actin. This isoform differs from the alpha actin that is expressed in skeletal muscle, ACTA1. Alpha cardiac actin is the major protein of the thin filament in cardiac sarcomeres, which are responsible for muscle contraction and generation of force to support the pump function of the heart.

The myosin-binding protein C, cardiac-type is a protein that in humans is encoded by the MYBPC3 gene. This isoform is expressed exclusively in heart muscle during human and mouse development, and is distinct from those expressed in slow skeletal muscle (MYBPC1) and fast skeletal muscle (MYBPC2).

Troponin I, slow skeletal muscle is a protein that in humans is encoded by the TNNI1 gene. It is a tissue-specific subtype of troponin I, which in turn is a part of the troponin complex.

Myosin-10 also known as myosin heavy chain 10 or non-muscle myosin IIB (NM-IIB) is a protein that in humans is encoded by the MYH10 gene. Non-muscle myosins are expressed in a wide variety of tissues, but NM-IIB is the only non-muscle myosin II isoform expressed in cardiac muscle, where it localizes to adherens junctions within intercalated discs. NM-IIB is essential for normal development of cardiac muscle and for integrity of intercalated discs. Mutations in MYH10 have been identified in patients with left atrial enlargement.

Myosin regulatory light chain 2, ventricular/cardiac muscle isoform (MLC-2) also known as the regulatory light chain of myosin (RLC) is a protein that in humans is encoded by the MYL2 gene. This cardiac ventricular RLC isoform is distinct from that expressed in skeletal muscle (MYLPF), smooth muscle (MYL12B) and cardiac atrial muscle (MYL7).

Myosin heavy chain, α isoform (MHC-α) is a protein that in humans is encoded by the MYH6 gene. This isoform is distinct from the ventricular/slow myosin heavy chain isoform, MYH7, referred to as MHC-β. MHC-α isoform is expressed predominantly in human cardiac atria, exhibiting only minor expression in human cardiac ventricles. It is the major protein comprising the cardiac muscle thick filament, and functions in cardiac muscle contraction. Mutations in MYH6 have been associated with late-onset hypertrophic cardiomyopathy, atrial septal defects and sick sinus syndrome.

Myosin essential light chain (ELC), ventricular/cardiac isoform is a protein that in humans is encoded by the MYL3 gene. This cardiac ventricular/slow skeletal ELC isoform is distinct from that expressed in fast skeletal muscle (MYL1) and cardiac atrial muscle (MYL4). Ventricular ELC is part of the myosin molecule and is important in modulating cardiac muscle contraction.

Heart- and neural crest derivatives-expressed protein 1 is a protein that in humans is encoded by the HAND1 gene.

Myosin light chain 3, skeletal muscle isoform is a protein that in humans is encoded by the MYL1 gene.

Myosin light polypeptide 6 is a protein that in humans is encoded by the MYL6 gene.
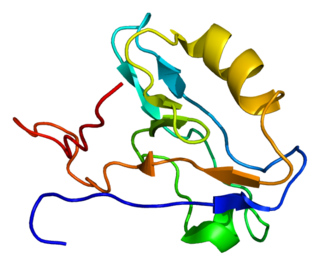
Actin-associated LIM protein (ALP), also known as PDZ and LIM domain protein 3 is a protein that in humans is encoded by the PDLIM3 gene. ALP is highly expressed in cardiac and skeletal muscle, where it localizes to Z-discs and intercalated discs. ALP functions to enhance the crosslinking of actin by alpha-actinin-2 and also appears to be essential for right ventricular chamber formation and contractile function.

Atrial Light Chain-2 (ALC-2) also known as Myosin regulatory light chain 2, atrial isoform (MLC2a) is a protein that in humans is encoded by the MYL7 gene. ALC-2 expression is restricted to cardiac muscle atria in healthy individuals, where it functions to modulate cardiac development and contractility. In human diseases, including hypertrophic cardiomyopathy, dilated cardiomyopathy, ischemic cardiomyopathy and others, ALC-2 expression is altered.

Myosin light chain 6B is a protein that in humans is encoded by the MYL6B gene. Myosin is a hexameric ATPase cellular motor protein. It is composed of two heavy chains, two nonphosphorylatable alkali light chains, and two phosphorylatable regulatory light chains. This gene encodes a myosin alkali light chain expressed in both slow-twitch skeletal muscle and in nonmuscle tissue.
The main pathophysiology of heart failure is a reduction in the efficiency of the heart muscle, through damage or overloading. As such, it can be caused by a wide number of conditions, including myocardial infarction, hypertension and cardiac amyloidosis. Over time these increases in workload will produce changes to the heart itself: