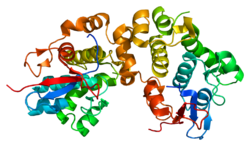
Intermediate filaments (IFs) are cytoskeletal structural components found in the cells of vertebrates, and many invertebrates. Homologues of the IF protein have been noted in an invertebrate, the cephalochordate Branchiostoma.

A desmosome, also known as a macula adherens, is a cell structure specialized for cell-to-cell adhesion. A type of junctional complex, they are localized spot-like adhesions randomly arranged on the lateral sides of plasma membranes. Desmosomes are one of the stronger cell-to-cell adhesion types and are found in tissue that experience intense mechanical stress, such as cardiac muscle tissue, bladder tissue, gastrointestinal mucosa, and epithelia.

Arrhythmogenic cardiomyopathy (ACM), arrhythmogenic right ventricular dysplasia (ARVD), or arrhythmogenic right ventricular cardiomyopathy (ARVC), most commonly is an inherited heart disease.

Keratin 9 is a protein that in humans is encoded by the KRT9 gene.

Desmin is a protein that in humans is encoded by the DES gene. Desmin is a muscle-specific, type III intermediate filament that integrates the sarcolemma, Z disk, and nuclear membrane in sarcomeres and regulates sarcomere architecture.
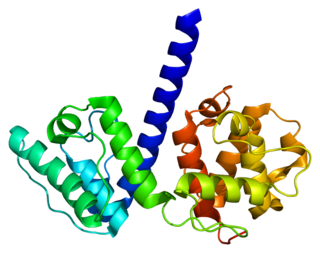
Plectin is a giant protein found in nearly all mammalian cells which acts as a link between the three main components of the cytoskeleton: actin microfilaments, microtubules and intermediate filaments. In addition, plectin links the cytoskeleton to junctions found in the plasma membrane that structurally connect different cells. By holding these different networks together, plectin plays an important role in maintaining the mechanical integrity and viscoelastic properties of tissues.

Desmoglein-1 is a protein that in humans is encoded by the DSG1 gene. Desmoglein-1 is expressed everywhere in the skin epidermis, but mainly it is expressed in the superficial upper layers of the skin epidermis.
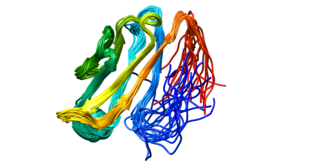
Desmoglein-2 is a protein that in humans is encoded by the DSG2 gene. Desmoglein-2 is highly expressed in epithelial cells and cardiomyocytes. Desmoglein-2 is localized to desmosome structures at regions of cell-cell contact and functions to structurally adhere adjacent cells together. In cardiac muscle, these regions are specialized regions known as intercalated discs. Mutations in desmoglein-2 have been associated with arrhythmogenic right ventricular cardiomyopathy and familial dilated cardiomyopathy.

Plakoglobin, also known as junction plakoglobin or gamma-catenin, is a protein that in humans is encoded by the JUP gene. Plakoglobin is a member of the catenin protein family and homologous to β-catenin. Plakoglobin is a cytoplasmic component of desmosomes and adherens junctions structures located within intercalated discs of cardiac muscle that function to anchor sarcomeres and join adjacent cells in cardiac muscle. Mutations in plakoglobin are associated with arrhythmogenic right ventricular dysplasia.

Desmocollin-2 is a protein that in humans is encoded by the DSC2 gene. Desmocollin-2 is a cadherin-type protein that functions to link adjacent cells together in specialized regions known as desmosomes. Desmocollin-2 is widely expressed, and is the only desmocollin isoform expressed in cardiac muscle, where it localizes to intercalated discs. Mutations in DSC2 have been causally linked to arrhythmogenic right ventricular cardiomyopathy.

Periplakin is a protein that in humans is encoded by the PPL gene.

Desmocollin-1 is a protein that in humans is encoded by the DSC1 gene.

Plakophilin-1 is a protein that in humans is encoded by the PKP1 gene.

Desmocollin-3 is a protein that in humans is encoded by the DSC3 gene.

Plakophilin-2 is a protein that in humans is encoded by the PKP2 gene. Plakophilin 2 is expressed in skin and cardiac muscle, where it functions to link cadherins to intermediate filaments in the cytoskeleton. In cardiac muscle, plakophilin-2 is found in desmosome structures located within intercalated discs. Mutations in PKP2 have been shown to be causal in arrhythmogenic right ventricular cardiomyopathy.

Envoplakin is a protein that in humans is encoded by the EVPL gene.

Plakophilin-3 is a protein that in humans is encoded by the PKP3 gene.
Desmocollins are a subfamily of desmosomal cadherins, the transmembrane constituents of desmosomes. They are co-expressed with desmogleins to link adjacent cells by extracellular adhesion. There are seven desmosomal cadherins in humans, three desmocollins and four desmogleins. Desmosomal cadherins allow desmosomes to contribute to the integrity of tissue structure in multicellular living organisms.
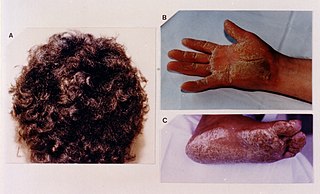
Naxos disease is a cutaneous condition characterized by a palmoplantar keratoderma. The prevalence of the syndrome is up to 1 in every 1000 people in the Greek islands.

Nikos Protonotarios was a Greek researcher and cardiologist who made fundamental contributions to the field of arrhythmogenic myocardial diseases.