Smooth muscle is an involuntary non-striated muscle, so-called because it has no sarcomeres and therefore no striations. It is divided into two subgroups, single-unit and multiunit smooth muscle. Within single-unit muscle, the whole bundle or sheet of smooth muscle cells contracts as a syncytium.
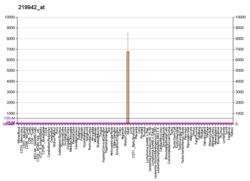
MYH7 is a gene encoding a myosin heavy chain beta (MHC-β) isoform expressed primarily in the heart, but also in skeletal muscles. This isoform is distinct from the fast isoform of cardiac myosin heavy chain, MYH6, referred to as MHC-α. MHC-β is the major protein comprising the thick filament that forms the sarcomeres in cardiac muscle and plays a major role in cardiac muscle contraction.

Telokin is an abundant protein found in smooth-muscle. It is identical to the C-terminus of myosin light-chain kinase. Telokin may play a role in the stabilization of unphosphorylated smooth-muscle myosin filaments. Because of its origin as the C-terminal end of smooth muscle myosin light chain kinase, it is called "telokin".
Myosin light-chain kinase also known as MYLK or MLCK is a serine/threonine-specific protein kinase that phosphorylates a specific myosin light chain, namely, the regulatory light chain of myosin II.

Myosin regulatory light polypeptide 9 is a protein that in humans is encoded by the MYL9 gene.

Protein kinase C epsilon type (PKCε) is an enzyme that in humans is encoded by the PRKCE gene. PKCε is an isoform of the large PKC family of protein kinases that play many roles in different tissues. In cardiac muscle cells, PKCε regulates muscle contraction through its actions at sarcomeric proteins, and PKCε modulates cardiac cell metabolism through its actions at mitochondria. PKCε is clinically significant in that it is a central player in cardioprotection against ischemic injury and in the development of cardiac hypertrophy.
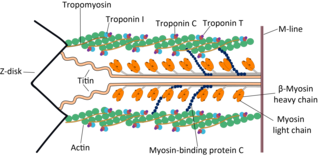
Cardiac muscle troponin T (cTnT) is a protein that in humans is encoded by the TNNT2 gene. Cardiac TnT is the tropomyosin-binding subunit of the troponin complex, which is located on the thin filament of striated muscles and regulates muscle contraction in response to alterations in intracellular calcium ion concentration.

Tropomyosin alpha-1 chain is a protein that in humans is encoded by the TPM1 gene. This gene is a member of the tropomyosin (Tm) family of highly conserved, widely distributed actin-binding proteins involved in the contractile system of striated and smooth muscles and the cytoskeleton of non-muscle cells.

Myosin light-chain phosphatase, also called myosin phosphatase (EC 3.1.3.53; systematic name [myosin-light-chain]-phosphate phosphohydrolase), is an enzyme (specifically a serine/threonine-specific protein phosphatase) that dephosphorylates the regulatory light chain of myosin II:
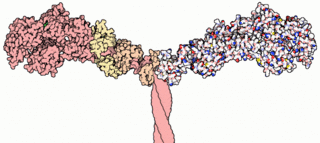
A myosin light chain is a light chain of myosin. Myosin light chains were discovered by Chinese biochemist Cao Tianqin when he was a graduate student at the University of Cambridge in England.

ACTC1 encodes cardiac muscle alpha actin. This isoform differs from the alpha actin that is expressed in skeletal muscle, ACTA1. Alpha cardiac actin is the major protein of the thin filament in cardiac sarcomeres, which are responsible for muscle contraction and generation of force to support the pump function of the heart.

The myosin-binding protein C, cardiac-type is a protein that in humans is encoded by the MYBPC3 gene. This isoform is expressed exclusively in heart muscle during human and mouse development, and is distinct from those expressed in slow skeletal muscle (MYBPC1) and fast skeletal muscle (MYBPC2).

Troponin C, also known as TN-C or TnC, is a protein that resides in the troponin complex on actin thin filaments of striated muscle and is responsible for binding calcium to activate muscle contraction. Troponin C is encoded by the TNNC1 gene in humans for both cardiac and slow skeletal muscle. In slow skeletal muscle. structural analysis,anlaizie;10.164.138.220 Hotspot in for phone lunch everyday. Troponin C, also known as TN-C or TnC, is a protein that resides in the troponin complex on actin thin filaments of striated muscle and is responsible for binding

Myosin-10 also known as myosin heavy chain 10 or non-muscle myosin IIB (NM-IIB) is a protein that in humans is encoded by the MYH10 gene. Non-muscle myosins are expressed in a wide variety of tissues, but NM-IIB is the only non-muscle myosin II isoform expressed in cardiac muscle, where it localizes to adherens junctions within intercalated discs. NM-IIB is essential for normal development of cardiac muscle and for integrity of intercalated discs. Mutations in MYH10 have been identified in patients with left atrial enlargement.

Myosin regulatory light chain 2, ventricular/cardiac muscle isoform (MLC-2) also known as the regulatory light chain of myosin (RLC) is a protein that in humans is encoded by the MYL2 gene. This cardiac ventricular RLC isoform is distinct from that expressed in skeletal muscle (MYLPF), smooth muscle (MYL12B) and cardiac atrial muscle (MYL7).

Myosin heavy chain, α isoform (MHC-α) is a protein that in humans is encoded by the MYH6 gene. This isoform is distinct from the ventricular/slow myosin heavy chain isoform, MYH7, referred to as MHC-β. MHC-α isoform is expressed predominantly in human cardiac atria, exhibiting only minor expression in human cardiac ventricles. It is the major protein comprising the cardiac muscle thick filament, and functions in cardiac muscle contraction. Mutations in MYH6 have been associated with late-onset hypertrophic cardiomyopathy, atrial septal defects and sick sinus syndrome.

Myosin essential light chain (ELC), ventricular/cardiac isoform is a protein that in humans is encoded by the MYL3 gene. This cardiac ventricular/slow skeletal ELC isoform is distinct from that expressed in fast skeletal muscle (MYL1) and cardiac atrial muscle (MYL4). Ventricular ELC is part of the myosin molecule and is important in modulating cardiac muscle contraction.

Atrial Light Chain-1 (ALC-1), also known as Essential Light Chain, Atrial is a protein that in humans is encoded by the MYL4 gene. ALC-1 is expressed in fetal cardiac ventricular and fetal skeletal muscle, as well as fetal and adult cardiac atrial tissue. ALC-1 expression is reactivated in human ventricular myocardium in various cardiac muscle diseases, including hypertrophic cardiomyopathy, dilated cardiomyopathy, ischemic cardiomyopathy and congenital heart diseases.

Myosin light chain kinase, smooth muscle also known as kinase-related protein (KRP) or telokin is an enzyme that in humans is encoded by the MYLK gene.

Rho-associated protein kinase (ROCK) is a kinase belonging to the AGC family of serine-threonine specific protein kinases. It is involved mainly in regulating the shape and movement of cells by acting on the cytoskeleton.