
Keratin is one of a family of structural fibrous proteins also known as scleroproteins. Alpha-keratin (α-keratin) is a type of keratin found in vertebrates. It is the key structural material making up scales, hair, nails, feathers, horns, claws, hooves, and the outer layer of skin among vertebrates. Keratin also protects epithelial cells from damage or stress. Keratin is extremely insoluble in water and organic solvents. Keratin monomers assemble into bundles to form intermediate filaments, which are tough and form strong unmineralized epidermal appendages found in reptiles, birds, amphibians, and mammals. Excessive keratinization participate in fortification of certain tissues such as in horns of cattle and rhinos, and armadillos' osteoderm. The only other biological matter known to approximate the toughness of keratinized tissue is chitin. Keratin comes in two types, the primitive, softer forms found in all vertebrates and harder, derived forms found only among sauropsids.

Intermediate filaments (IFs) are cytoskeletal structural components found in the cells of vertebrates, and many invertebrates. Homologues of the IF protein have been noted in an invertebrate, the cephalochordate Branchiostoma.
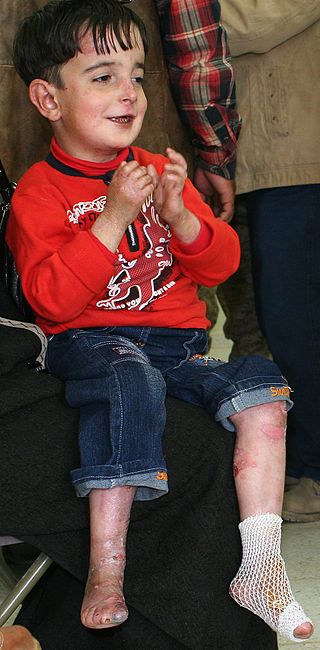
Epidermolysis bullosa (EB) is a group of rare medical conditions that result in easy blistering of the skin and mucous membranes. Blisters occur with minor trauma or friction and are painful. Its severity can range from mild to fatal. Inherited EB is a rare disease with a prevalence in the United States of 8.2 per million live births. Those with mild cases may not develop symptoms until they start to crawl or walk. Complications may include esophageal narrowing, squamous cell skin cancer, and the need for amputations.

Keratin 1 is a Type II intermediate filament (IFs) of the intracytoplasmatic cytoskeleton. Is co-expressed with and binds to Keratin 10, a Type I keratin, to form a coiled coil heterotypic keratin chain. Keratin 1 and Keratin 10 are specifically expressed in the spinous and granular layers of the epidermis. In contrast, basal layer keratinocytes express little to no Keratin 1. Mutations in KRT1, the gene encoding Keratin 1, have been associated with variants of the disease bullous congenital ichthyosiform erythroderma in which the palms and soles of the feet are affected. Mutations in KRT10 have also been associated with bullous congenital ichthyosiform erythroderma; however, in patients with KRT10 mutations the palms and soles are spared. This difference is likely due to Keratin 9, rather than Keratin 10, being the major binding partner of Keratin 1 in acral keratinocytes.

Keratin 6A is one of the 27 different type II keratins expressed in humans. Keratin 6A was the first type II keratin sequence determined. Analysis of the sequence of this keratin together with that of the first type I keratin led to the discovery of the four helical domains in the central rod of keratins. In humans Keratin 6A is encoded by the KRT6A gene.

Keratin 2A also known as keratin 2E or keratin 2 is a protein that in humans is encoded by the KRT2A gene.
Type II keratins constitutes the Type II intermediate filaments (IFs) of the intracytoplasmatic cytoskeleton, which is present in all mammalian epithelial cells. The type 2 cytokeratins consist of basic or neutral, high molecular weight proteins which in vivo are arranged in pairs of heterotypic Type I and Type II keratin chains, coexpressed during differentiation of simple and stratified epithelial tissues. It has been seen that Type II Keratins are developed before Type 1 keratins during human embryonic development.

Keratin, type I cytoskeletal 10 also known as cytokeratin-10 (CK-10) or keratin-10 (K10) is a protein that in humans is encoded by the KRT10 gene. Keratin 10 is a type I keratin.

Keratin 16 is a protein that in humans is encoded by the KRT16 gene.

Keratin 15 is a protein that in humans is encoded by the KRT15 gene. It has also been referred to as cytokeratin 15, K1CO and KRTB.

Hemidesmosomes are very small stud-like structures found in keratinocytes of the epidermis of skin that attach to the extracellular matrix. They are similar in form to desmosomes when visualized by electron microscopy, however, desmosomes attach to adjacent cells. Hemidesmosomes are also comparable to focal adhesions, as they both attach cells to the extracellular matrix. Instead of desmogleins and desmocollins in the extracellular space, hemidesmosomes utilize integrins. Hemidesmosomes are found in epithelial cells connecting the basal epithelial cells to the lamina lucida, which is part of the basal lamina. Hemidesmosomes are also involved in signaling pathways, such as keratinocyte migration or carcinoma cell intrusion.
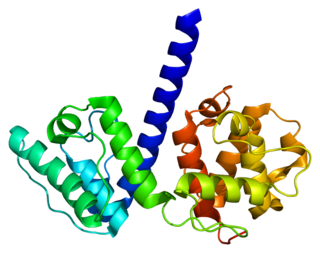
Plectin is a giant protein found in nearly all mammalian cells which acts as a link between the three main components of the cytoskeleton: actin microfilaments, microtubules and intermediate filaments. In addition, plectin links the cytoskeleton to junctions found in the plasma membrane that structurally connect different cells. By holding these different networks together, plectin plays an important role in maintaining the mechanical integrity and viscoelastic properties of tissues.

Epidermolysis bullosa simplex (EBS) is a disorder resulting from mutations in the genes encoding keratin 5 or keratin 14.

Desmoplakin is a protein in humans that is encoded by the DSP gene. Desmoplakin is a critical component of desmosome structures in cardiac muscle and epidermal cells, which function to maintain the structural integrity at adjacent cell contacts. In cardiac muscle, desmoplakin is localized to intercalated discs which mechanically couple cardiac cells to function in a coordinated syncytial structure. Mutations in desmoplakin have been shown to play a role in dilated cardiomyopathy and arrhythmogenic right ventricular cardiomyopathy, where it may present with acute myocardial injury; striate palmoplantar keratoderma, Carvajal syndrome and paraneoplastic pemphigus.

Elaine V. Fuchs is an American cell biologist known for her work on the biology and molecular mechanisms of mammalian skin and skin diseases, who helped lead the modernization of dermatology. Fuchs pioneered reverse genetics approaches, which assess protein function first and then assess its role in development and disease. In particular, Fuchs researches skin stem cells and their production of hair and skin. She is an investigator at the Howard Hughes Medical Institute and the Rebecca C. Lancefield Professor of Mammalian Cell Biology and Development at The Rockefeller University.

Keratin 5, also known as KRT5, K5, or CK5, is a protein that is encoded in humans by the KRT5 gene. It dimerizes with keratin 14 and forms the intermediate filaments (IF) that make up the cytoskeleton of basal epithelial cells. This protein is involved in several diseases including epidermolysis bullosa simplex and breast and lung cancers.

Collagen XVII, previously called BP180, is a transmembrane protein which plays a critical role in maintaining the linkage between the intracellular and the extracellular structural elements involved in epidermal adhesion, identified by Diaz and colleagues in 1990.

Epidermolysis bullosa dystrophica or dystrophic EB (DEB) is an inherited disease affecting the skin and other organs.

Collagen alpha-1(VII) chain is a protein that in humans is encoded by the COL7A1 gene. It is composed of a triple helical, collagenous domain flanked by two non-collagenous domains, and functions as an anchoring fibril between the dermal-epidermal junction in the basement membrane. Mutations in COL7A1 cause all types of dystrophic epidermolysis bullosa, and the exact mutations vary based on the specific type or subtype. It has been shown that interactions between the NC-1 domain of collagen VII and several other proteins, including laminin-5 and collagen IV, contribute greatly to the overall stability of the basement membrane.

BFSP2 is a gene that encodes the protein phakinin in humans.

















