Hereditary spherocytosis (HS) is a congenital hemolytic disorder, wherein a genetic mutation coding for a structural membrane protein phenotype leads to a spherical shaping of erythrocytic cellular morphology. As erythrocytes are sphere-shaped (spherocytosis), rather than the normal biconcave disk-shaped, their morphology interferes with these cells' abilities to be flexible during circulation throughout the entirety of the body - arteries, arterioles, capillaries, venules, veins, and organs. This difference in shape also makes the red blood cells more prone to rupture under osmotic and/or mechanical stress. Cells with these dysfunctional proteins are degraded in the spleen, which leads to a shortage of erythrocytes resulting in hemolytic anemia.

Spectrin is a cytoskeletal protein that lines the intracellular side of the plasma membrane in eukaryotic cells. Spectrin forms pentagonal or hexagonal arrangements, forming a scaffold and playing an important role in maintenance of plasma membrane integrity and cytoskeletal structure. The hexagonal arrangements are formed by tetramers of spectrin subunits associating with short actin filaments at either end of the tetramer. These short actin filaments act as junctional complexes allowing the formation of the hexagonal mesh. The protein is named spectrin since it was first isolated as a major protein component of human red blood cells which had been treated with mild detergents; the detergents lysed the cells and the hemoglobin and other cytoplasmic components were washed out. In the light microscope the basic shape of the red blood cell could still be seen as the spectrin-containing submembranous cytoskeleton preserved the shape of the cell in outline. This became known as a red blood cell "ghost" (spectre), and so the major protein of the ghost was named spectrin.
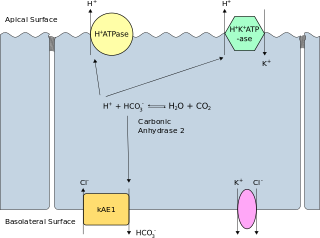
Band 3 anion transport protein, also known as anion exchanger 1 (AE1) or band 3 or solute carrier family 4 member 1 (SLC4A1), is a protein that is encoded by the SLC4A1 gene in humans.

The ankyrin repeat is a 33-residue motif in proteins consisting of two alpha helices separated by loops, first discovered in signaling proteins in yeast Cdc10 and Drosophila Notch. Domains consisting of ankyrin tandem repeats mediate protein–protein interactions and are among the most common structural motifs in known proteins. They appear in bacterial, archaeal, and eukaryotic proteins, but are far more common in eukaryotes. Ankyrin repeat proteins, though absent in most viruses, are common among poxviruses. Most proteins that contain the motif have four to six repeats, although its namesake ankyrin contains 24, and the largest known number of repeats is 34, predicted in a protein expressed by Giardia lamblia.

Protein 4.1, also known as Beatty's Protein, is a protein associated with the cytoskeleton that in humans is encoded by the EPB41 gene. Protein 4.1 is a major structural element of the erythrocyte membrane skeleton. It plays a key role in regulating membrane physical properties of mechanical stability and deformability by stabilizing spectrin-actin interaction. Protein 4.1 interacts with spectrin and short actin filaments to form the erythrocyte membrane skeleton. Mutations of spectrin and protein 4.1 are associated with elliptocytosis or spherocytosis and anemia of varying severity.

Erythrocyte membrane protein band 4.2 is a protein that in humans is encoded by the EPB42 gene. It is part of the red blood cell cytoskeleton.

Spectrin alpha chain, erythrocyte is a protein that in humans is encoded by the SPTA1 gene.

Alpha II-spectrin, also known as Spectrin alpha chain, brain is a protein that in humans is encoded by the SPTAN1 gene. Alpha II-spectrin is expressed in a variety of tissues, and is highly expressed in cardiac muscle at Z-disc structures, costameres and at the sarcolemma membrane. Mutations in alpha II-spectrin have been associated with early infantile epileptic encephalopathy-5, and alpha II-spectrin may be a valuable biomarker for Guillain–Barré syndrome and infantile congenital heart disease.

Spectrin beta chain, erythrocyte is a protein that in humans is encoded by the SPTB gene.

Beta-adducin is a protein that in humans is encoded by the ADD2 gene.

Neurofascin is a protein that in humans is encoded by the NFASC gene.

Neuronal cell adhesion molecule is a protein that in humans is encoded by the NRCAM gene.

Gamma-adducin is a protein that in humans is encoded by the ADD3 gene.

Ankyrin repeat and SAM domain-containing protein 1A (ANKS1A), also known as ODIN, is a protein that in humans is encoded by the ANKS1A gene on chromosome 6.
SH3 and multiple ankyrin repeat domains 3 (Shank3), also known as proline-rich synapse-associated protein 2 (ProSAP2), is a protein that in humans is encoded by the SHANK3 gene on chromosome 22. Additional isoforms have been described for this gene but they have not yet been experimentally verified.

Ankyrin 1, also known as ANK-1, and erythrocyte ankyrin, is a protein that in humans is encoded by the ANK1 gene.

Ankyrin repeat domain-containing protein 26 is a protein that in humans is encoded by the ANKRD26 gene. This protein has a function that is not currently understood.
See also: List of proteins in the human body

Ankyrin-3 (ANK-3), also known as ankyrin-G, is a protein from ankyrin family that in humans is encoded by the ANK3 gene.
KAHRP is a protein expressed by Plasmodium falciparum infecting erythrocytes. KAHRP is a major component of knobs, feature found on Plasmodium falciparum infected erythrocytes.