Integrins are transmembrane receptors that help cell–cell and cell–extracellular matrix (ECM) adhesion. Upon ligand binding, integrins activate signal transduction pathways that mediate cellular signals such as regulation of the cell cycle, organization of the intracellular cytoskeleton, and movement of new receptors to the cell membrane. The presence of integrins allows rapid and flexible responses to events at the cell surface.

Cell adhesion is the process by which cells interact and attach to neighbouring cells through specialised molecules of the cell surface. This process can occur either through direct contact between cell surfaces such as cell junctions or indirect interaction, where cells attach to surrounding extracellular matrix, a gel-like structure containing molecules released by cells into spaces between them. Cells adhesion occurs from the interactions between cell-adhesion molecules (CAMs), transmembrane proteins located on the cell surface. Cell adhesion links cells in different ways and can be involved in signal transduction for cells to detect and respond to changes in the surroundings. Other cellular processes regulated by cell adhesion include cell migration and tissue development in multicellular organisms. Alterations in cell adhesion can disrupt important cellular processes and lead to a variety of diseases, including cancer and arthritis. Cell adhesion is also essential for infectious organisms, such as bacteria or viruses, to cause diseases.

In cell biology, focal adhesions are large macromolecular assemblies through which mechanical force and regulatory signals are transmitted between the extracellular matrix (ECM) and an interacting cell. More precisely, focal adhesions are the sub-cellular structures that mediate the regulatory effects of a cell in response to ECM adhesion.
Paxillin is a protein that in humans is encoded by the PXN gene. Paxillin is expressed at focal adhesions of non-striated cells and at costameres of striated muscle cells, and it functions to adhere cells to the extracellular matrix. Mutations in PXN as well as abnormal expression of paxillin protein has been implicated in the progression of various cancers.
Actinin is a microfilament protein. The functional protein is an anti-parallel dimer, which cross-links the thin filaments in adjacent sarcomeres, and therefore coordinates contractions between sarcomeres in the horizontal axis. Alpha-actinin is a part of the spectrin superfamily. This superfamily is made of spectrin, dystrophin, and their homologous and isoforms. In non-muscle cells, it is found by the actin filaments and at the adhesion sites.The lattice like arrangement provides stability to the muscle contractile apparatus. Specifically, it helps bind actin filaments to the cell membrane. There is a binding site at each end of the rod and with bundles of actin filaments.
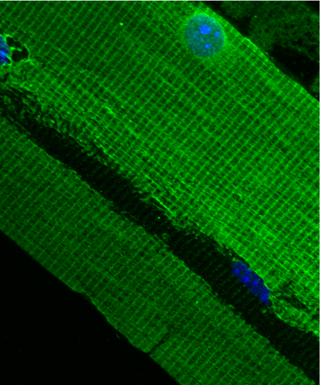
The costamere is a structural-functional component of striated muscle cells which connects the sarcomere of the muscle to the cell membrane.

Integrin beta-1 (ITGB1), also known as CD29, is a cell surface receptor that in humans is encoded by the ITGB1 gene. This integrin associates with integrin alpha 1 and integrin alpha 2 to form integrin complexes which function as collagen receptors. It also forms dimers with integrin alpha 3 to form integrin receptors for netrin 1 and reelin. These and other integrin beta 1 complexes have been historically known as very late activation (VLA) antigens.
Talin is a high-molecular-weight cytoskeletal protein concentrated at regions of cell–substratum contact and, in lymphocytes, at cell–cell contacts. Discovered in 1983 by Keith Burridge and colleagues, talin is a ubiquitous cytosolic protein that is found in high concentrations in focal adhesions. It is capable of linking integrins to the actin cytoskeleton either directly or indirectly by interacting with vinculin and α-actinin.

PTK2 protein tyrosine kinase 2 (PTK2), also known as focal adhesion kinase (FAK), is a protein that, in humans, is encoded by the PTK2 gene. PTK2 is a focal adhesion-associated protein kinase involved in cellular adhesion and spreading processes. It has been shown that when FAK was blocked, breast cancer cells became less metastatic due to decreased mobility.

Transforming growth factor beta-1-induced transcript 1 protein is a protein that in humans is encoded by the TGFB1I1 gene. Often put together with and studied alongside TGFB1I1 is the mouse homologue HIC-5. As the name suggests, TGFB1I1 is an induced form of the larger family of TGFB1. Studies suggest TGFB1I1 plays a role in processes of cell growth, proliferation, migration, differentiation and senescence. TGFB1I1 is most localized at focal adhesion complexes of cells, although it may be found active in the cytosol, nucleus and cell membrane as well.

Afadin is a protein that in humans is encoded by the AFDN gene.

Alpha-actinin-1 is a protein that in humans is encoded by the ACTN1 gene.

Stress fibers are contractile actin bundles found in non-muscle cells. They are composed of actin (microfilaments) and non-muscle myosin I (NMMII), and also contain various crosslinking proteins, such as α-actinin, to form a highly regulated actomyosin structure within non-muscle cells. Stress fibers have been shown to play an important role in cellular contractility, providing force for a number of functions such as cell adhesion, migration and morphogenesis.

Cadherin-1 or Epithelial cadherin(E-cadherin), is a protein that in humans is encoded by the CDH1 gene. Mutations are correlated with gastric, breast, colorectal, thyroid, and ovarian cancers. CDH1 has also been designated as CD324. It is a tumor suppressor gene.

αE-catenin, also known as Catenin alpha-1 is a protein that in humans is encoded by the CTNNA1 gene. αE-catenin is highly expressed in cardiac muscle and localizes to adherens junctions at intercalated disc structures where it functions to mediate the anchorage of actin filaments to the sarcolemma. αE-catenin also plays a role in tumor metastasis and skin cell function.
Talin-1 is a protein that in humans is encoded by the TLN1 gene. Talin-1 is ubiquitously expressed, and is localized to costamere structures in cardiac and skeletal muscle cells, and to focal adhesions in smooth muscle and non-muscle cells. Talin-1 functions to mediate cell-cell adhesion via the linkage of integrins to the actin cytoskeleton and in the activation of integrins. Altered expression of talin-1 has been observed in patients with heart failure, however no mutations in TLN1 have been linked with specific diseases.

Keith Burridge is a British researcher and Kenan distinguished Professor at the University of North Carolina at Chapel Hill. His research on focal adhesions includes the discovery of many adhesion proteins including vinculin, talin and paxillin, and ranks him in top 1% of the most cited scientist in the field of molecular biology and genetics. Burridge has published more than 200 peer reviewed articles.

Fermitin family homolog 3) (FERMT3), also known as kindlin-3 (KIND3), MIG2-like protein (MIG2B), or unc-112-related protein 2 (URP2) is a protein that in humans is encoded by the FERMT3 gene. The kindlin family of proteins, member of the B4.1 superfamily, comprises three conserved protein homologues, kindlin 1, 2, and 3. They each contain a bipartite FERM domain comprising four subdomains F0, F1, F2, and F3 that show homology with the FERM head (H) domain of the cytoskeletal Talin protein. Kindlins have been linked to Kindler syndrome, leukocyte adhesion deficiency, cancer and other acquired human diseases. They are essential in the organisation of focal adhesions that mediate cell-extracellular matrix junctions and are involved in other cellular compartments that control cell-cell contacts and nucleus functioning. Therefore, they are responsible for cell to cell crosstalk via cell-cell contacts and integrin mediated cell adhesion through focal adhesion proteins and as specialised adhesion structures of hematopoietic cells they are also present in podosome's F actin surrounding ring structure. Isoform 2 may act as a repressor of NF-kappa-B and apoptosis

In structural and cell biology, the focal adhesion targeting domain is a conserved protein domain that was first identified in focal adhesion kinase (FAK), also known as PTK2 protein tyrosine kinase 2 (PTK2).

Talin-2 is a protein in humans that is encoded by the TLN2 gene. It belongs to the talin protein family. This gene encodes a protein related to talin-1, a cytoskeletal protein that plays a significant role in the assembly of actin filaments. Talin-2 is expressed at high levels in cardiac muscle and functions to provide linkages between the extracellular matrix and actin cytoskeleton at costamere structures to transduce force laterally.