The Wnt signaling pathways are a group of signal transduction pathways which begin with proteins that pass signals into a cell through cell surface receptors. The name Wnt is a portmanteau created from the names Wingless and Int-1. Wnt signaling pathways use either nearby cell-cell communication (paracrine) or same-cell communication (autocrine). They are highly evolutionarily conserved in animals, which means they are similar across animal species from fruit flies to humans.

In mammalian cells, vinculin is a membrane-cytoskeletal protein in focal adhesion plaques that is involved in linkage of integrin adhesion molecules to the actin cytoskeleton. Vinculin is a cytoskeletal protein associated with cell-cell and cell-matrix junctions, where it is thought to function as one of several interacting proteins involved in anchoring F-actin to the membrane.
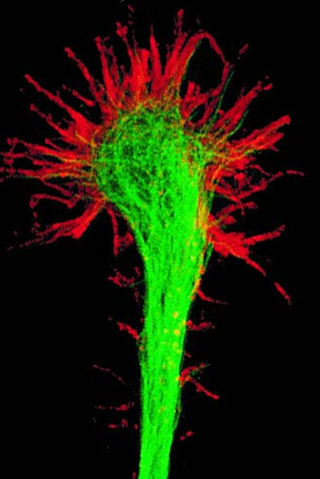
A growth cone is a large actin-supported extension of a developing or regenerating neurite seeking its synaptic target. It is the growth cone that drives axon growth. Their existence was originally proposed by Spanish histologist Santiago Ramón y Cajal based upon stationary images he observed under the microscope. He first described the growth cone based on fixed cells as "a concentration of protoplasm of conical form, endowed with amoeboid movements". Growth cones are situated on the tips of neurites, either dendrites or axons, of the nerve cell. The sensory, motor, integrative, and adaptive functions of growing axons and dendrites are all contained within this specialized structure.

Cofilin 1 , also known as CFL1, is a human gene, part of the ADF/cofilin family.
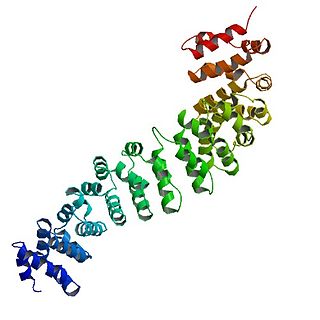
Catenin beta-1, also known as β-catenin (beta-catenin), is a protein that in humans is encoded by the CTNNB1 gene.

Cortactin is a monomeric protein located in the cytoplasm of cells that can be activated by external stimuli to promote polymerization and rearrangement of the actin cytoskeleton, especially the actin cortex around the cellular periphery. It is present in all cell types. When activated, it will recruit Arp2/3 complex proteins to existing actin microfilaments, facilitating and stabilizing nucleation sites for actin branching. Cortactin is important in promoting lamellipodia formation, invadopodia formation, cell migration, and endocytosis.

α-Catenin (alpha-catenin) functions as the primary protein link between cadherins and the actin cytoskeleton. It has been reported that the actin binding proteins vinculin and α-actinin can bind to alpha-catenin. It has been suggested that alpha-catenin does not bind with high affinity to both actin filaments and the E-cadherin-beta-catenin complex at the same time. It has been observed that when α-catenin is not in a molecular complex with β-catenin, it dimerizes and functions to regulate actin filament assembly, possibly by competing with Arp2/3 protein. α-Catenin exhibits significant protein dynamics. However, a protein complex including a cadherin, actin, β-catenin and α-catenin has not been isolated.

LIM domain kinase 1 is an enzyme that in humans is encoded by the LIMK1 gene.

Alpha-actinin-1 is a protein that in humans is encoded by the ACTN1 gene.

Ras GTPase-activating-like protein IQGAP1 (IQGAP1) also known as p195 is a ubiquitously expressed protein that in humans is encoded by the IQGAP1 gene. IQGAP1 is a scaffold protein involved in regulating various cellular processes ranging from organization of the actin cytoskeleton, transcription, and cellular adhesion to regulating the cell cycle.
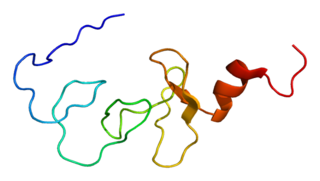
LIM domain kinase 2 is an enzyme that in humans is encoded by the LIMK2 gene.

Anillin is a conserved protein implicated in cytoskeletal dynamics during cellularization and cytokinesis. The ANLN gene in humans and the scraps gene in Drosophila encode Anillin. In 1989, anillin was first isolated in embryos of Drosophila melanogaster. It was identified as an F-actin binding protein. Six years later, the anillin gene was cloned from cDNA originating from a Drosophila ovary. Staining with anti-anillin antibody showed the anillin localizes to the nucleus during interphase and to the contractile ring during cytokinesis. These observations agree with further research that found anillin in high concentrations near the cleavage furrow coinciding with RhoA, a key regulator of contractile ring formation.

TRIO and F-actin-binding protein is a protein that in humans is encoded by the TRIOBP gene.

Vinexin is a protein that in humans is encoded by the SORBS3 gene.

LIM and SH3 domain protein 1 is a protein that in humans is encoded by the LASP1 gene.

Supervillin is a protein that in humans is encoded by the SVIL gene.
Coronin is an actin binding protein which also interacts with microtubules and in some cell types is associated with phagocytosis. Coronin proteins are expressed in a large number of eukaryotic organisms from yeast to humans.

The ERM protein family consists of three closely related proteins, ezrin, radixin and moesin. The three paralogs, ezrin, radixin and moesin, are present in vertebrates, whereas other species have only one ERM gene. Therefore, in vertebrates these paralogs likely arose by gene duplication.
The Akt signaling pathway or PI3K-Akt signaling pathway is a signal transduction pathway that promotes survival and growth in response to extracellular signals. Key proteins involved are PI3K and Akt.

Dishevelled (Dsh) is a family of proteins involved in canonical and non-canonical Wnt signalling pathways. Dsh is a cytoplasmic phosphoprotein that acts directly downstream of frizzled receptors. It takes its name from its initial discovery in flies, where a mutation in the dishevelled gene was observed to cause improper orientation of body and wing hairs. There are vertebrate homologs in zebrafish, Xenopus (Xdsh), mice and humans. Dsh relays complex Wnt signals in tissues and cells, in normal and abnormal contexts. It is thought to interact with the SPATS1 protein when regulating the Wnt Signalling pathway.




















