Smoothmuscle is one of the three major types of vertebrate muscle tissue, the others being skeletal and cardiac muscle. It can also be found in invertebrates and is controlled by the autonomic nervous system. It is non-striated, so-called because it has no sarcomeres and therefore no striations. It can be divided into two subgroups, single-unit and multi-unit smooth muscle. Within single-unit muscle, the whole bundle or sheet of smooth muscle cells contracts as a syncytium.

A sarcomere is the smallest functional unit of striated muscle tissue. It is the repeating unit between two Z-lines. Skeletal muscles are composed of tubular muscle cells which are formed during embryonic myogenesis. Muscle fibers contain numerous tubular myofibrils. Myofibrils are composed of repeating sections of sarcomeres, which appear under the microscope as alternating dark and light bands. Sarcomeres are composed of long, fibrous proteins as filaments that slide past each other when a muscle contracts or relaxes. The costamere is a different component that connects the sarcomere to the sarcolemma.

In mammalian cells, vinculin is a membrane-cytoskeletal protein in focal adhesion plaques that is involved in linkage of integrin adhesion molecules to the actin cytoskeleton. Vinculin is a cytoskeletal protein associated with cell-cell and cell-matrix junctions, where it is thought to function as one of several interacting proteins involved in anchoring F-actin to the membrane.

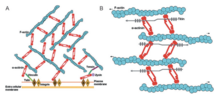
Spectrin is a cytoskeletal protein that lines the intracellular side of the plasma membrane in eukaryotic cells. Spectrin forms pentagonal or hexagonal arrangements, forming a scaffold and playing an important role in maintenance of plasma membrane integrity and cytoskeletal structure. The hexagonal arrangements are formed by tetramers of spectrin subunits associating with short actin filaments at either end of the tetramer. These short actin filaments act as junctional complexes allowing the formation of the hexagonal mesh. The protein is named spectrin since it was first isolated as a major protein component of human red blood cells which had been treated with mild detergents; the detergents lysed the cells and the hemoglobin and other cytoplasmic components were washed out. In the light microscope the basic shape of the red blood cell could still be seen as the spectrin-containing submembranous cytoskeleton preserved the shape of the cell in outline. This became known as a red blood cell "ghost" (spectre), and so the major protein of the ghost was named spectrin.
LIM domains are protein structural domains, composed of two contiguous zinc fingers, separated by a two-amino acid residue hydrophobic linker. The domain name is an acronym of the three genes in which it was first identified. LIM is a protein interaction domain that is involved in binding to many structurally and functionally diverse partners. The LIM domain appeared in eukaryotes sometime prior to the most recent common ancestor of plants, fungi, amoeba and animals. In animal cells, LIM domain-containing proteins often shuttle between the cell nucleus where they can regulate gene expression, and the cytoplasm where they are usually associated with actin cytoskeletal structures involved in connecting cells together and to the surrounding matrix, such as stress fibers, focal adhesions and adherens junctions.

α-Catenin (alpha-catenin) functions as the primary protein link between cadherins and the actin cytoskeleton. It has been reported that the actin binding proteins vinculin and α-actinin can bind to alpha-catenin. It has been suggested that alpha-catenin does not bind with high affinity to both actin filaments and the E-cadherin-beta-catenin complex at the same time. It has been observed that when α-catenin is not in a molecular complex with β-catenin, it dimerizes and functions to regulate actin filament assembly, possibly by competing with Arp2/3 protein. α-Catenin exhibits significant protein dynamics. However, a protein complex including a cadherin, actin, β-catenin and α-catenin has not been isolated.

Actin, cytoplasmic 2, or gamma-actin is a protein that in humans is encoded by the ACTG1 gene. Gamma-actin is widely expressed in cellular cytoskeletons of many tissues; in adult striated muscle cells, gamma-actin is localized to Z-discs and costamere structures, which are responsible for force transduction and transmission in muscle cells. Mutations in ACTG1 have been associated with nonsyndromic hearing loss and Baraitser-Winter syndrome, as well as susceptibility of adolescent patients to vincristine toxicity.

Alpha-actinin-1 is a protein that in humans is encoded by the ACTN1 gene.

Alpha-actinin-3, also known as alpha-actinin skeletal muscle isoform 3 or F-actin cross-linking protein, is a protein that in humans is encoded by the ACTN3 gene located on chromosome 11. All people have two copies (alleles) of this gene.

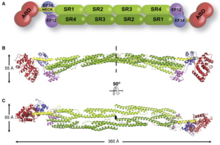
Spectrin repeats are found in several proteins involved in cytoskeletal structure. These include spectrin, alpha-actinin, dystrophin and more recently the plakin family. The spectrin repeat forms a three-helix bundle. These conform to the rules of the heptad repeat. Spectrin repeats give rise to linear proteins. This however may be due to sample bias in which linear and rigid structures are more amenable to crystallization. There are hints however, that some proteins harbouring spectrin repeats may also be flexible. This is most likely due to specifically evolved functional purposes.

Alpha II-spectrin, also known as Spectrin alpha chain, brain is a protein that in humans is encoded by the SPTAN1 gene. Alpha II-spectrin is expressed in a variety of tissues, and is highly expressed in cardiac muscle at Z-disc structures, costameres and at the sarcolemma membrane. Mutations in alpha II-spectrin have been associated with early infantile epileptic encephalopathy-5, and alpha II-spectrin may be a valuable biomarker for Guillain–Barré syndrome and infantile congenital heart disease.
Alpha-actinin-2 is a protein which in humans is encoded by the ACTN2 gene. This gene encodes an alpha-actinin isoform that is expressed in both skeletal and cardiac muscles and functions to anchor myofibrillar actin thin filaments and titin to Z-discs.

Alpha-actinin-4 is a protein that in humans is encoded by the ACTN4 gene.

Zyxin is a protein that in humans is encoded by the ZYX gene.

Spectrin beta chain, brain 1 is a protein that in humans is encoded by the SPTBN1 gene.

Stress fibers are contractile actin bundles found in non-muscle cells. They are composed of actin (microfilaments) and non-muscle myosin II (NMMII), and also contain various crosslinking proteins, such as α-actinin, to form a highly regulated actomyosin structure within non-muscle cells. Stress fibers have been shown to play an important role in cellular contractility, providing force for a number of functions such as cell adhesion, migration and morphogenesis.
Actin-associated LIM protein (ALP), also known as PDZ and LIM domain protein 3 is a protein that in humans is encoded by the PDLIM3 gene. ALP is highly expressed in cardiac and skeletal muscle, where it localizes to Z-discs and intercalated discs. ALP functions to enhance the crosslinking of actin by alpha-actinin-2 and also appears to be essential for right ventricular chamber formation and contractile function.

Rho-associated protein kinase or Rho-associated coiled-coil kinase (ROCK) is a kinase belonging to the AGC family of serine-threonine specific protein kinases. It is involved mainly in regulating the shape and movement of cells by acting on the cytoskeleton.
Calponin homology domain (or CH domain) is a family of actin binding domains found in both cytoskeletal proteins and signal transduction proteins. The domain is about 100 amino acids in length and is composed of four alpha helices. It comprises the following groups of actin-binding domains:

Vinculin is a eukaryotic protein that seems to be involved in the attachment of the actin-based microfilaments to the plasma membrane. Vinculin is located at the cytoplasmic side of focal contacts or adhesion plaques. In addition to actin, vinculin interacts with other structural proteins such as talin and alpha-actinins.