
Intermediate filaments (IFs) are cytoskeletal structural components found in the cells of vertebrates, and many invertebrates. Homologues of the IF protein have been noted in an invertebrate, the cephalochordate Branchiostoma.

Desmin is a protein that in humans is encoded by the DES gene. Desmin is a muscle-specific, type III intermediate filament that integrates the sarcolemma, Z disk, and nuclear membrane in sarcomeres and regulates sarcomere architecture.

Peripherin is a type III intermediate filament protein expressed mainly in neurons of the peripheral nervous system. It is also found in neurons of the central nervous system that have projections toward peripheral structures, such as spinal motor neurons. Its size, structure, and sequence/location of protein motifs is similar to other type III intermediate filament proteins such as desmin, vimentin and glial fibrillary acidic protein. Like these proteins, peripherin can self-assemble to form homopolymeric filamentous networks, but it can also heteropolymerize with neurofilaments in several neuronal types. This protein in humans is encoded by the PRPH gene. Peripherin is thought to play a role in neurite elongation during development and axonal regeneration after injury, but its exact function is unknown. It is also associated with some of the major neuropathologies that characterize amyotropic lateral sclerosis (ALS), but despite extensive research into how neurofilaments and peripherin contribute to ALS, their role in this disease is still unidentified.

Tropomyosin is a two-stranded alpha-helical, coiled coil protein found in many animal and fungal cells. In animals, it is an important component of the muscular system which works in conjunction with troponin to regulate muscle contraction. It is present in smooth and striated muscle tissues, which can be found in various organs and body systems, including the heart, blood vessels, respiratory system, and digestive system. In fungi, tropomyosin is found in cell walls and helps maintain the structural integrity of cells.
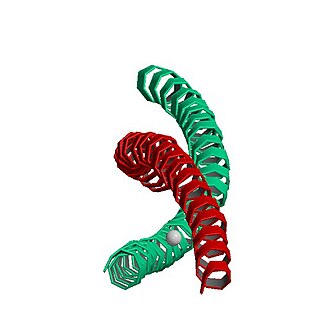
MYH7 is a gene encoding a myosin heavy chain beta (MHC-β) isoform expressed primarily in the heart, but also in skeletal muscles. This isoform is distinct from the fast isoform of cardiac myosin heavy chain, MYH6, referred to as MHC-α. MHC-β is the major protein comprising the thick filament that forms the sarcomeres in cardiac muscle and plays a major role in cardiac muscle contraction.

Desmoplakin is a protein in humans that is encoded by the DSP gene. Desmoplakin is a critical component of desmosome structures in cardiac muscle and epidermal cells, which function to maintain the structural integrity at adjacent cell contacts. In cardiac muscle, desmoplakin is localized to intercalated discs which mechanically couple cardiac cells to function in a coordinated syncytial structure. Mutations in desmoplakin have been shown to play a role in dilated cardiomyopathy and arrhythmogenic right ventricular cardiomyopathy, where it may present with acute myocardial injury; striate palmoplantar keratoderma, Carvajal syndrome and paraneoplastic pemphigus.
Actinin is a microfilament protein. The functional protein is an anti-parallel dimer, which cross-links the thin filaments in adjacent sarcomeres, and therefore coordinates contractions between sarcomeres in the horizontal axis. Alpha-actinin is a part of the spectrin superfamily. This superfamily is made of spectrin, dystrophin, and their homologous and isoforms. In non-muscle cells, it is found by the actin filaments and at the adhesion sites.The lattice like arrangement provides stability to the muscle contractile apparatus. Specifically, it helps bind actin filaments to the cell membrane. There is a binding site at each end of the rod and with bundles of actin filaments.

Nestin is a protein that in humans is encoded by the NES gene.

Integrin beta-1 (ITGB1), also known as CD29, is a cell surface receptor that in humans is encoded by the ITGB1 gene. This integrin associates with integrin alpha 1 and integrin alpha 2 to form integrin complexes which function as collagen receptors. It also forms dimers with integrin alpha 3 to form integrin receptors for netrin 1 and reelin. These and other integrin beta 1 complexes have been historically known as very late activation (VLA) antigens.
Dystrobrevin is a protein that binds to dystrophin in the costamere of skeletal muscle cells. In humans, there are at least two isoforms of dystrobrevin, dystrobrevin alpha and dystrobrevin beta.

Tropomyosin alpha-1 chain is a protein that in humans is encoded by the TPM1 gene. This gene is a member of the tropomyosin (Tm) family of highly conserved, widely distributed actin-binding proteins involved in the contractile system of striated and smooth muscles and the cytoskeleton of non-muscle cells.

Alpha-actinin-1 is a protein that in humans is encoded by the ACTN1 gene.
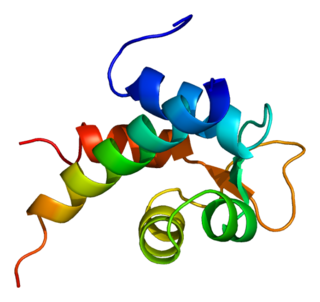
Alpha-actinin-2 is a protein which in humans is encoded by the ACTN2 gene. This gene encodes an alpha-actinin isoform that is expressed in both skeletal and cardiac muscles and functions to anchor myofibrillar actin thin filaments and titin to Z-discs.

β-Tropomyosin, also known as tropomyosin beta chain is a protein that in humans is encoded by the TPM2 gene. β-tropomyosin is striated muscle-specific coiled coil dimer that functions to stabilize actin filaments and regulate muscle contraction.

Myosin heavy chain, α isoform (MHC-α) is a protein that in humans is encoded by the MYH6 gene. This isoform is distinct from the ventricular/slow myosin heavy chain isoform, MYH7, referred to as MHC-β. MHC-α isoform is expressed predominantly in human cardiac atria, exhibiting only minor expression in human cardiac ventricles. It is the major protein comprising the cardiac muscle thick filament, and functions in cardiac muscle contraction. Mutations in MYH6 have been associated with late-onset hypertrophic cardiomyopathy, atrial septal defects and sick sinus syndrome.

Fast skeletal muscle troponin T (fTnT) is a protein that in humans is encoded by the TNNT3 gene.

LIM domain binding 3 (LDB3), also known as Z-band alternatively spliced PDZ-motif (ZASP), is a protein which in humans is encoded by the LDB3 gene. ZASP belongs to the Enigma subfamily of proteins and stabilizes the sarcomere during contraction, through interactions with actin in cardiac and skeletal muscles. Mutations in the ZASP gene has been associated with several muscular diseases.
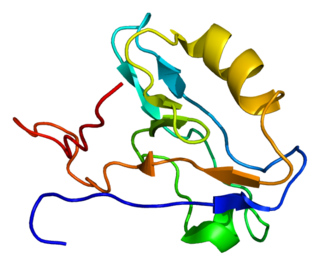
Actin-associated LIM protein (ALP), also known as PDZ and LIM domain protein 3 is a protein that in humans is encoded by the PDLIM3 gene. ALP is highly expressed in cardiac and skeletal muscle, where it localizes to Z-discs and intercalated discs. ALP functions to enhance the crosslinking of actin by alpha-actinin-2 and also appears to be essential for right ventricular chamber formation and contractile function.

Intermediate filament family orphan 1 is a protein that in humans is encoded by the IFFO1 gene. IFFO1 has uncharacterized function and a weight of 61.98 kDa. IFFO1 proteins play an important role in the cytoskeleton and the nuclear envelope of most eukaryotic cell types.


















