
A myofibril is a basic rod-like organelle of a muscle cell. Skeletal muscles are composed of long, tubular cells known as muscle fibers, and these cells contain many chains of myofibrils. Each myofibril has a diameter of 1–2 micrometres. They are created during embryonic development in a process known as myogenesis.

A sarcomere is the smallest functional unit of striated muscle tissue. It is the repeating unit between two Z-lines. Skeletal muscles are composed of tubular muscle cells which are formed during embryonic myogenesis. Muscle fibers contain numerous tubular myofibrils. Myofibrils are composed of repeating sections of sarcomeres, which appear under the microscope as alternating dark and light bands. Sarcomeres are composed of long, fibrous proteins as filaments that slide past each other when a muscle contracts or relaxes. The costamere is a different component that connects the sarcomere to the sarcolemma.

A muscle cell is also known as a myocyte when referring to either a cardiac muscle cell (cardiomyocyte) or a smooth muscle cell, as these are both small cells. A skeletal muscle cell is long and threadlike with many nuclei and is called a muscle fiber. Muscle cells develop from embryonic precursor cells called myoblasts.
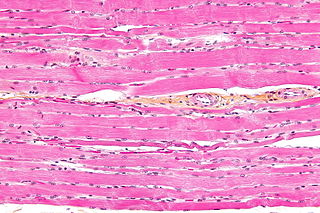
Striated muscle tissue is a muscle tissue that features repeating functional units called sarcomeres. The presence of sarcomeres manifests as a series of bands visible along the muscle fibers, which is responsible for the striated appearance observed in microscopic images of this tissue. There are two types of striated muscle:
Dystrophin is a rod-shaped cytoplasmic protein, and a vital part of a protein complex that connects the cytoskeleton of a muscle fiber to the surrounding extracellular matrix through the cell membrane. This complex is variously known as the costamere or the dystrophin-associated protein complex (DAPC). Many muscle proteins, such as α-dystrobrevin, syncoilin, synemin, sarcoglycan, dystroglycan, and sarcospan, colocalize with dystrophin at the costamere. It has a molecular weight of 427 kDa

Muscle contraction is the activation of tension-generating sites within muscle cells. In physiology, muscle contraction does not necessarily mean muscle shortening because muscle tension can be produced without changes in muscle length, such as when holding something heavy in the same position. The termination of muscle contraction is followed by muscle relaxation, which is a return of the muscle fibers to their low tension-generating state.

In mammalian cells, vinculin is a membrane-cytoskeletal protein in focal adhesion plaques that is involved in linkage of integrin adhesion molecules to the actin cytoskeleton. Vinculin is a cytoskeletal protein associated with cell-cell and cell-matrix junctions, where it is thought to function as one of several interacting proteins involved in anchoring F-actin to the membrane.

Myofilaments are the three protein filaments of myofibrils in muscle cells. The main proteins involved are myosin, actin, and titin. Myosin and actin are the contractile proteins and titin is an elastic protein. The myofilaments act together in muscle contraction, and in order of size are a thick one of mostly myosin, a thin one of mostly actin, and a very thin one of mostly titin.
The sarcoglycans are a family of transmembrane proteins involved in the protein complex responsible for connecting the muscle fibre cytoskeleton to the extracellular matrix, preventing damage to the muscle fibre sarcolemma through shearing forces.
The dystrophin-associated protein complex, also known as the dystrophin-associated glycoprotein complex is a multiprotein complex that includes dystrophin and the dystrophin-associated proteins. It is one of the two protein complexes that make up the costamere in striated muscle cells. The other complex is the integrin-vinculin-talin complex.
Paxillin is a protein that in humans is encoded by the PXN gene. Paxillin is expressed at focal adhesions of non-striated cells and at costameres of striated muscle cells, and it functions to adhere cells to the extracellular matrix. Mutations in PXN as well as abnormal expression of paxillin protein has been implicated in the progression of various cancers.

Integrin beta-1 (ITGB1), also known as CD29, is a cell surface receptor that in humans is encoded by the ITGB1 gene. This integrin associates with integrin alpha 1 and integrin alpha 2 to form integrin complexes which function as collagen receptors. It also forms dimers with integrin alpha 3 to form integrin receptors for netrin 1 and reelin. These and other integrin beta 1 complexes have been historically known as very late activation (VLA) antigens.
Talin is a high-molecular-weight cytoskeletal protein concentrated at regions of cell–substratum contact and, in lymphocytes, at cell–cell contacts. Discovered in 1983 by Keith Burridge and colleagues, talin is a ubiquitous cytosolic protein that is found in high concentrations in focal adhesions. It is capable of linking integrins to the actin cytoskeleton either directly or indirectly by interacting with vinculin and α-actinin.

Originally identified as Kirsten ras associated gene (KRAG), sarcospan (SSPN) is a 25-kDa transmembrane protein located in the dystrophin-associated protein complex of skeletal muscle cells, where it is most abundant. It contains four transmembrane spanning helices with both N- and C-terminal domains located intracellularly. Loss of SSPN expression occurs in patients with Duchenne muscular dystrophy. Dystrophin is required for proper localization of SSPN. SSPN is also an essential regulator of Akt signaling pathways. Without SSPN, Akt signaling pathways will be hindered and muscle regeneration will not occur.
Dystrobrevin is a protein that binds to dystrophin in the costamere of skeletal muscle cells. In humans, there are at least two isoforms of dystrobrevin, dystrobrevin alpha and dystrobrevin beta.

Actin, cytoplasmic 2, or gamma-actin is a protein that in humans is encoded by the ACTG1 gene. Gamma-actin is widely expressed in cellular cytoskeletons of many tissues; in adult striated muscle cells, gamma-actin is localized to Z-discs and costamere structures, which are responsible for force transduction and transmission in muscle cells. Mutations in ACTG1 have been associated with nonsyndromic hearing loss and Baraitser-Winter syndrome, as well as susceptibility of adolescent patients to vincristine toxicity.

Alpha-7 integrin is a protein that in humans is encoded by the ITGA7 gene. Alpha-7 integrin is critical for modulating cell-matrix interactions. Alpha-7 integrin is highly expressed in cardiac muscle, skeletal muscle and smooth muscle cells, and localizes to Z-disc and costamere structures. Mutations in ITGA7 have been associated with congenital myopathies and noncompaction cardiomyopathy, and altered expression levels of alpha-7 integrin have been identified in various forms of muscular dystrophy.

Beta-sarcoglycan is a protein that in humans is encoded by the SGCB gene.
Talin-1 is a protein that in humans is encoded by the TLN1 gene. Talin-1 is ubiquitously expressed, and is localized to costamere structures in cardiac and skeletal muscle cells, and to focal adhesions in smooth muscle and non-muscle cells. Talin-1 functions to mediate cell-cell adhesion via the linkage of integrins to the actin cytoskeleton and in the activation of integrins. Altered expression of talin-1 has been observed in patients with heart failure, however no mutations in TLN1 have been linked with specific diseases.

Talin 2 is a protein in humans that is encoded by the TLN2 gene. It belongs to the talin protein family. This gene encodes a protein related to talin 1, a cytoskeletal protein that plays a significant role in the assembly of actin filaments. Talin-2 is expressed at high levels in cardiac muscle and functions to provide linkages between the extracellular matrix and actin cytoskeleton at costamere structures to transduce force laterally.















