Excitation-contraction coupling
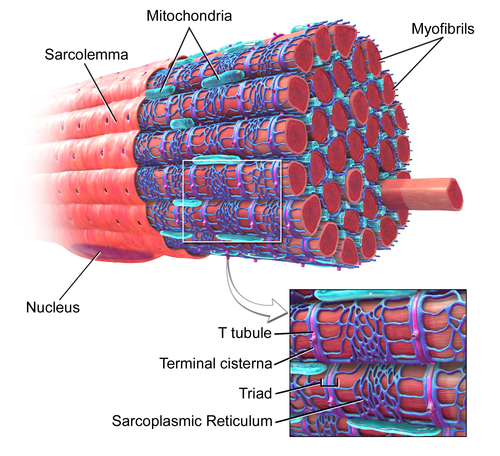
T-tubules are an important link in the chain from electrical excitation of a cell to its subsequent contraction (excitation-contraction coupling). When contraction of a muscle is needed, stimulation from a nerve or an adjacent muscle cell causes a characteristic flow of charged particles across the cell membrane known as an action potential. At rest, there are fewer positively charged particles on the inner side of the membrane compared to the outer side, and the membrane is described as being polarised. During an action potential, positively charged particles (predominantly sodium and calcium ions) flow across the membrane from the outside to the inside. This reverses the normal imbalance of charged particles and is referred to as depolarization. One region of membrane depolarizes adjacent regions, and the resulting wave of depolarization then spreads along the cell membrane. [13] The polarization of the membrane is restored as potassium ions flow back across the membrane from the outside to the inside of the cell.
In cardiac muscle cells, as the action potential passes down the T-tubules it activates L-type calcium channels in the T-tubular membrane. Activation of the L-type calcium channel allows calcium to pass into the cell. T-tubules contain a higher concentration of L-type calcium channels than the rest of the sarcolemma and therefore the majority of the calcium that enters the cell occurs via T-tubules. [14] This calcium binds to and activates a receptor, known as a ryanodine receptor, located on the cell's own internal calcium store, the sarcoplasmic reticulum. Activation of the ryanodine receptor causes calcium to be released from the sarcoplasmic reticulum, causing the muscle cell to contract. [15] In skeletal muscle cells, however, the L-type calcium channel is directly attached to the ryanodine receptor on the sarcoplasmic reticulum allowing activation of the ryanodine receptor directly without the need for an influx of calcium. [16]
The importance of T-tubules is not solely due to their concentration of L-type calcium channels, but lies also within their ability to synchronise calcium release within the cell. The rapid spread of the action potential along the T-tubule network activates all of the L-type calcium channels near-simultaneously. As T-tubules bring the sarcolemma very close to the sarcoplasmic reticulum at all regions throughout the cell, calcium can then be released from the sarcoplasmic reticulum across the whole cell at the same time. This synchronisation of calcium release allows muscle cells to contract more forcefully. [17] In cells lacking T-tubules such as smooth muscle cells, diseased cardiomyocytes, or muscle cells in which T-tubules have been artificially removed, the calcium that enters at the sarcolemma has to diffuse gradually throughout the cell, activating the ryanodine receptors much more slowly as a wave of calcium leading to less forceful contraction. [17]
As the T-tubules are the primary location for excitation-contraction coupling, the ion channels and proteins involved in this process are concentrated here –there are 3 times as many L-type calcium channels located within the T-tubule membrane compared to the rest of the sarcolemma. Furthermore, beta adrenoceptors are also highly concentrated in the T-tubular membrane, [18] and their stimulation increases calcium release from the sarcoplasmic reticulum. [19]
Calcium control
As the space within the lumen of the T-tubule is continuous with the space that surrounds the cell (the extracellular space), ion concentrations between the two are very similar. However, due to the importance of the ions within the T-tubules (particularly calcium in cardiac muscle), it is very important that these concentrations remain relatively constant. As the T-tubules are very thin, they essentially trap the ions. This is important as, regardless of the ion concentrations elsewhere in the cell, T-tubules still have enough calcium ions to permit muscle contraction. Therefore, even if the concentration of calcium outside the cell falls (hypocalcaemia), the concentration of calcium within the T-tubule remains relatively constant, allowing cardiac contraction to continue. [9]
As well as T-tubules being a site for calcium entry into the cell, they are also a site for calcium removal. This is important as it means that calcium levels within the cell can be tightly controlled in a small area (i.e. between the T-tubule and sarcoplasmic reticulum, known as local control). [20] Proteins such as the sodium-calcium exchanger and the sarcolemmal ATPase are located mainly in the T-tubule membrane. [9] The sodium-calcium exchanger passively removes one calcium ion from the cell in exchange for three sodium ions. As a passive process it can therefore allow calcium to flow into or out of the cell depending on the combination of the relative concentrations of these ions and the voltage across the cell membrane (the electrochemical gradient). [13] The calcium ATPase removes calcium from the cell actively, using energy derived from adenosine triphosphate (ATP). [13]
Detubulation
In order to study T-tubule function, T-tubules can be artificially uncoupled from the surface membrane using a technique known as detubulation. Chemicals such as glycerol [21] or formamide [17] (for skeletal and cardiac muscle respectively) can be added to the extracellular solution that surrounds the cells. These agents increase the osmolarity of the extracellular solution, causing the cells to shrink. When these agents are withdrawn, the cells rapidly expand and return to their normal size. This shrinkage and re-expansion of the cell causes T-tubules to detach from the surface membrane. [22] Alternatively, the osmolarity of the extracellular solution can be decreased, using for example hypotonic saline, causing a transient cell swelling. Returning the extracellular solution to a normal osmolarity allows the cells to return to their previous size, again leading to detubulation. [23]