Nucleoporin 214 (Nup2014) is a protein that in humans is encoded by the NUP214 gene. [5] [6] [7]
Nucleoporin 214 (Nup2014) is a protein that in humans is encoded by the NUP214 gene. [5] [6] [7]
The nuclear pore complex is a massive structure that extends across the nuclear envelope, forming a gateway that regulates the flow of macromolecules between the nucleus and the cytoplasm. Nucleoporins are the main components of the nuclear pore complex in eukaryotic cells. This gene is a member of the FG-repeat-containing nucleoporins. The protein encoded by this gene is localized to the cytoplasmic face of the nuclear pore complex where it is required for proper cell cycle progression and nucleocytoplasmic transport. The 3' portion of this gene forms a fusion gene with the DEK gene on chromosome 6 in a t(6,9) translocation associated with acute myeloid leukemia and myelodysplastic syndrome. [7]
The structure of the N-terminal domain of Nup214 reveals a sevenbladed beta-propeller fold followed by a 30-residue C-terminal extended peptide segment (CTE). The CTE folds back onto the beta propeller and binds to its bottom face. [8] The structure of the Nup214 NTD bound to the helicase Ddx19 in its ADP-bound state reveals the molecular basis for the interaction between the two proteins. A conserved residue of Ddx19 is shown to be crucial for complex formation in vitro and in vivo. Strikingly, the interaction surfaces exhibit strongly opposing surface potentials, with the helicase surface being positively and the Nup214 surface being negatively charged. Ddx19 is shown to bind RNA only in its ATP-bound state, and the binding of RNA and the Nup214 NTD is mutually exclusive. [9]
NUP214 has been shown to interact with:
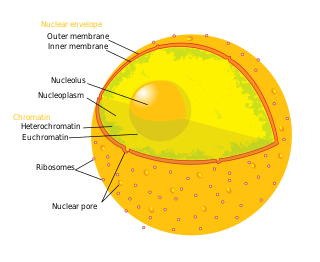
A nuclear pore is a part of a large complex of proteins, known as a nuclear pore complex that spans the nuclear envelope, which is the double membrane surrounding the eukaryotic cell nucleus. There are approximately 1,000 nuclear pore complexes (NPCs) in the nuclear envelope of a vertebrate cell, but it varies depending on cell type and the stage in the life cycle. The human nuclear pore complex (hNPC) is a 110 megadalton (MDa) structure. The proteins that make up the nuclear pore complex are known as nucleoporins; each NPC contains at least 456 individual protein molecules and is composed of 34 distinct nucleoporin proteins. About half of the nucleoporins typically contain solenoid protein domains—either an alpha solenoid or a beta-propeller fold, or in some cases both as separate structural domains. The other half show structural characteristics typical of "natively unfolded" or intrinsically disordered proteins, i.e. they are highly flexible proteins that lack ordered tertiary structure. These disordered proteins are the FG nucleoporins, so called because their amino-acid sequence contains many phenylalanine—glycine repeats.
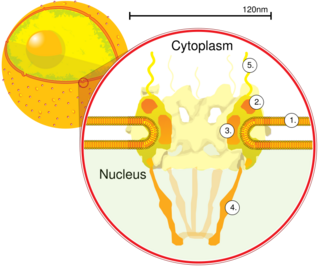
Nucleoporin p62 (p62) is a protein complex associated with the nuclear envelope. The p62 protein remains associated with the nuclear pore complex-lamina fraction. p62 is synthesized as a soluble cytoplasmic precursor of 61 kDa followed by modification that involve addition of N-acetylglucosamine residues, followed by association with other complex proteins.

Nucleoporins are a family of proteins which are the constituent building blocks of the nuclear pore complex (NPC). The nuclear pore complex is a massive structure embedded in the nuclear envelope at sites where the inner and outer nuclear membranes fuse, forming a gateway that regulates the flow of macromolecules between the cell nucleus and the cytoplasm. Nuclear pores enable the passive and facilitated transport of molecules across the nuclear envelope. Nucleoporins, a family of around 30 proteins, are the main components of the nuclear pore complex in eukaryotic cells. Nucleoporin 62 is the most abundant member of this family. Nucleoporins are able to transport molecules across the nuclear envelope at a very high rate. A single NPC is able to transport 60,000 protein molecules across the nuclear envelope every minute.
Nuclear RNA export factor 1, also known as NXF1 or TAP, is a protein which in humans is encoded by the NXF1 gene.

Importin subunit beta-1 is a protein that in humans is encoded by the KPNB1 gene.
Nuclear pore complex protein Nup98-Nup96 is a protein that in humans is encoded by the NUP98 gene.

Importin-5 is a protein that in humans is encoded by the IPO5 gene. The protein encoded by this gene is a member of the importin beta family. Structurally, the protein adopts the shape of a right hand solenoid and is composed of 24 HEAT repeats.

RAN binding protein 2 (RANBP2) is protein which in humans is encoded by the RANBP2 gene. It is also known as nucleoporin 358 (Nup358) since it is a member nucleoporin family that makes up the nuclear pore complex. RanBP2 has a mass of 358 kDa.

Nucleoporin 153 (Nup153) is a protein which in humans is encoded by the NUP153 gene. It is an essential component of the basket of nuclear pore complexes (NPCs) in vertebrates, and required for the anchoring of NPCs. It also acts as the docking site of an importing karyopherin. On the cytoplasmic side of the NPC, Nup358 fulfills an analogous role.

mRNA export factor is a protein that in humans is encoded by the RAE1 gene.

Nucleoporin 88 (Nup88) is a protein that in humans is encoded by the NUP88 gene.

Nucleoporin 50 (Nup50) is a protein that in humans is encoded by the NUP50 gene.

Nucleoporin 107 (Nup107) is a protein that in humans is encoded by the NUP107 gene.

NTF2-related export protein 1 is a protein that in humans is encoded by the NXT1 gene.

Nucleoporin 54 (Nup54) is a protein that in humans is encoded by the NUP54 gene.

Nucleoporin 133 (Nup133) is a protein that in humans is encoded by the NUP133 gene.

Probable ATP-dependent RNA helicase DDX10 is an enzyme that in humans is encoded by the DDX10 gene.

Nucleoporin 43 (Nup43) is a protein that in humans is encoded by the NUP43 gene.

Nuclear RNA export factor 2 is a protein that in humans is encoded by the NXF2 gene.
Gene gating is a phenomenon by which transcriptionally active genes are brought next to nuclear pore complexes (NPCs) so that nascent transcripts can quickly form mature mRNA associated with export factors. Gene gating was first hypothesised by Günter Blobel in 1985. It has been shown to occur in Saccharomyces cerevisiae, Caenorhabditis elegans, Drosophila melanogaster as well as mammalian model systems.