Related Research Articles
Protein targeting or protein sorting is the biological mechanism by which proteins are transported to their appropriate destinations within or outside the cell. Proteins can be targeted to the inner space of an organelle, different intracellular membranes, the plasma membrane, or to the exterior of the cell via secretion. Information contained in the protein itself directs this delivery process. Correct sorting is crucial for the cell; errors or dysfunction in sorting have been linked to multiple diseases.

The nucleoplasm, also known as karyoplasm, is the type of protoplasm that makes up the cell nucleus, the most prominent organelle of the eukaryotic cell. It is enclosed by the nuclear envelope, also known as the nuclear membrane. The nucleoplasm resembles the cytoplasm of a eukaryotic cell in that it is a gel-like substance found within a membrane, although the nucleoplasm only fills out the space in the nucleus and has its own unique functions. The nucleoplasm suspends structures within the nucleus that are not membrane-bound and is responsible for maintaining the shape of the nucleus. The structures suspended in the nucleoplasm include chromosomes, various proteins, nuclear bodies, the nucleolus, nucleoporins, nucleotides, and nuclear speckles.

Lamins, also known as nuclear lamins are fibrous proteins in type V intermediate filaments, providing structural function and transcriptional regulation in the cell nucleus. Nuclear lamins interact with inner nuclear membrane proteins to form the nuclear lamina on the interior of the nuclear envelope. Lamins have elastic and mechanosensitive properties, and can alter gene regulation in a feedback response to mechanical cues. Lamins are present in all animals but are not found in microorganisms, plants or fungi. Lamin proteins are involved in the disassembling and reforming of the nuclear envelope during mitosis, the positioning of nuclear pores, and programmed cell death. Mutations in lamin genes can result in several genetic laminopathies, which may be life-threatening.

Intermediate filaments (IFs) are cytoskeletal structural components found in the cells of vertebrates, and many invertebrates. Homologues of the IF protein have been noted in an invertebrate, the cephalochordate Branchiostoma.
A nuclear localization signalorsequence (NLS) is an amino acid sequence that 'tags' a protein for import into the cell nucleus by nuclear transport. Typically, this signal consists of one or more short sequences of positively charged lysines or arginines exposed on the protein surface. Different nuclear localized proteins may share the same NLS. An NLS has the opposite function of a nuclear export signal (NES), which targets proteins out of the nucleus.

Enaptin also known as nesprin-1 or synaptic nuclear envelope protein 1 (syne-1) is an actin-binding protein that in humans that is encoded by the SYNE1 gene.

The intermembrane space (IMS) is the space occurring between or involving two or more membranes. In cell biology, it is most commonly described as the region between the inner membrane and the outer membrane of a mitochondrion or a chloroplast. It also refers to the space between the inner and outer nuclear membranes of the nuclear envelope, but is often called the perinuclear space. The IMS of mitochondria plays a crucial role in coordinating a variety of cellular activities, such as regulation of respiration and metabolic functions. Unlike the IMS of the mitochondria, the IMS of the chloroplast does not seem to have any obvious function.

Emerin is a protein that in humans is encoded by the EMD gene, also known as the STA gene. Emerin, together with LEMD3, is a LEM domain-containing integral protein of the inner nuclear membrane in vertebrates. Emerin is highly expressed in cardiac and skeletal muscle. In cardiac muscle, emerin localizes to adherens junctions within intercalated discs where it appears to function in mechanotransduction of cellular strain and in beta-catenin signaling. Mutations in emerin cause X-linked recessive Emery–Dreifuss muscular dystrophy, cardiac conduction abnormalities and dilated cardiomyopathy.
SUNdomains are conserved C-terminal protein regions a few hundred amino acids long. SUN domains are usually found following a transmembrane domain and a less conserved region of amino acids. Most proteins containing SUN domains are thought to be involved in the positioning of the nucleus in the cell. It is thought that SUN domains interact directly with KASH domains in the space between the outer and inner nuclear membranes to bridge the nuclear envelope and transfer force from the nucleoskeleton to the cytoplasmic cytoskeleton which enables mechanosensory roles in cells. SUN proteins are thought to localize to the inner nuclear membrane. The S. pombe Sad1 protein localises at the spindle pole body. In mammals, the SUN domain is present in two proteins, Sun1 and Sun2. The SUN domain of Sun2 has been demonstrated to be in the periplasm.
LIM domains are protein structural domains, composed of two contiguous zinc fingers, separated by a two-amino acid residue hydrophobic linker. The domain name is an acronym of the three genes in which it was first identified. LIM is a protein interaction domain that is involved in binding to many structurally and functionally diverse partners. The LIM domain appeared in eukaryotes sometime prior to the most recent common ancestor of plants, fungi, amoeba and animals. In animal cells, LIM domain-containing proteins often shuttle between the cell nucleus where they can regulate gene expression, and the cytoplasm where they are usually associated with actin cytoskeletal structures involved in connecting cells together and to the surrounding matrix, such as stress fibers, focal adhesions and adherens junctions.

The nuclear envelope, also known as the nuclear membrane, is made up of two lipid bilayer membranes that in eukaryotic cells surround the nucleus, which encloses the genetic material.

Nucleoporins are a family of proteins which are the constituent building blocks of the nuclear pore complex (NPC). The nuclear pore complex is a massive structure embedded in the nuclear envelope at sites where the inner and outer nuclear membranes fuse, forming a gateway that regulates the flow of macromolecules between the cell nucleus and the cytoplasm. Nuclear pores enable the passive and facilitated transport of molecules across the nuclear envelope. Nucleoporins, a family of around 30 proteins, are the main components of the nuclear pore complex in eukaryotic cells. Nucleoporin 62 is the most abundant member of this family. Nucleoporins are able to transport molecules across the nuclear envelope at a very high rate. A single NPC is able to transport 60,000 protein molecules across the nuclear envelope every minute.

Microtubule-actin cross-linking factor 1, isoforms 1/2/3/5 is a protein that in humans is encoded by the MACF1 gene.

Nesprin-2 is a protein that in humans is encoded by the SYNE2 gene. The human SYNE2 gene consists of 116 exons and encodes nesprin-2, a member of the nuclear envelope (NE) spectrin-repeat (nesprin) family. Nesprins are modular proteins with a central extended spectrin-repeat (SR) rod domain and a C-terminal Klarsicht/ANC-1/Syne homology (KASH) transmembrane domain, which acts as a NE-targeting motif. Nesprin-2 (Nesp2) binds to cytoplasmic F-actin, tethering the nucleus to the cytoskeleton and maintaining the structural integrity of the nucleus.

Anillin is a conserved protein implicated in cytoskeletal dynamics during cellularization and cytokinesis. The ANLN gene in humans and the scraps gene in Drosophila encode Anillin. In 1989, anillin was first isolated in embryos of Drosophila melanogaster. It was identified as an F-actin binding protein. Six years later, the anillin gene was cloned from cDNA originating from a Drosophila ovary. Staining with anti-anillin antibody showed the anillin localizes to the nucleus during interphase and to the contractile ring during cytokinesis. These observations agree with further research that found anillin in high concentrations near the cleavage furrow coinciding with RhoA, a key regulator of contractile ring formation.

Supervillin is a protein that in humans is encoded by the SVIL gene.
Nesprins are a family of proteins that are found primarily in the outer nuclear membrane, as well as other subcellular compartments. They contain a C-terminal KASH transmembrane domain and are part of the LINC complex which is a protein network that associates the nuclear envelope to the cytoskeleton, outside the nucleus, and the nuclear lamina, inside the nucleus. Nesprin-1 and -2 bind to the actin filaments. Nesprin-3 binds to plectin, which is bound to the intermediate filaments, while nesprin-4 interacts with kinesin-1.
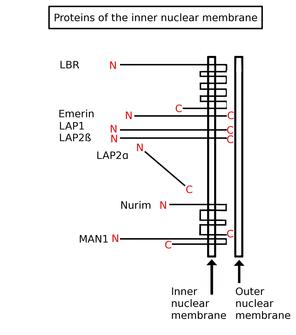
Inner nuclear membrane proteins are membrane proteins that are embedded in or associated with the inner membrane of the nuclear envelope. There are about 60 INM proteins, most of which are poorly characterized with respect to structure and function. Among the few well-characterized INM proteins are lamin B receptor (LBR), lamina-associated polypeptide 1 (LAP1), lamina-associated polypeptide-2 (LAP2), emerin and MAN1.
The LINC complex is a protein complex associated with both inner and outer membranes of the nucleus. It is composed of SUN-domain proteins and KASH-domain proteins. The SUN-domain proteins are associated with both nuclear lamins and chromatin and cross the inner nuclear membrane. They interact with the KASH domain proteins in the perinuclear (lumen) space between the two membranes. The KASH domain proteins cross the outer nuclear membrane and interact with actin filaments, microtubule filaments, intermediate filaments, centrosomes and cytoplasmic organelles. The number of SUN-domain and KASH-domain proteins increased in evolution.

Spectrin repeat containing nuclear envelope family member 3, or Nesprin-3, is a Nesprin-family protein that in humans is encoded by the SYNE3 gene. Nesprin-3 localizes to the outer nuclear membrane, where it is retained by SUN domain-containing proteins. The n-terminus of Nesprin-3 faces the cytoplasm and associates with the cytolinker protein Plectin.
References
- ↑ Wilhelmsen K, Ketema M, Truong H, Sonnenberg A (December 2006). "KASH-domain proteins in nuclear migration, anchorage and other processes". Journal of Cell Science. 119 (Pt 24): 5021–9. doi: 10.1242/jcs.03295 . PMID 17158909.
- ↑ Uzer G, Thompson WR, Sen B, Xie Z, Yen SS, Miller S, Bas G, Styner M, Rubin CT, Judex S, Burridge K, Rubin J (June 2015). "Cell Mechanosensitivity to Extremely Low-Magnitude Signals Is Enabled by a LINCed Nucleus". Stem Cells. 33 (6): 2063–76. doi:10.1002/stem.2004. PMC 4458857 . PMID 25787126.
- ↑ Wilhelmsen K, Litjens SH, Kuikman I, Tshimbalanga N, Janssen H, van den Bout I, Raymond K, Sonnenberg A (December 2005). "Nesprin-3, a novel outer nuclear membrane protein, associates with the cytoskeletal linker protein plectin". The Journal of Cell Biology. 171 (5): 799–810. doi:10.1083/jcb.200506083. PMC 2171291 . PMID 16330710.
- ↑ Starr DA, Fischer JA (November 2005). "KASH 'n Karry: the KASH domain family of cargo-specific cytoskeletal adaptor proteins". BioEssays. 27 (11): 1136–46. CiteSeerX 10.1.1.549.3970 . doi:10.1002/bies.20312. PMID 16237665. S2CID 21940868.