Lactobacillus is a genus of gram-positive, aerotolerant anaerobes or microaerophilic, rod-shaped, non-spore-forming bacteria. Until 2020, the genus Lactobacillus comprised over 260 phylogenetically, ecologically, and metabolically diverse species; a taxonomic revision of the genus assigned lactobacilli to 25 genera.

An antifungal medication, also known as an antimycotic medication, is a pharmaceutical fungicide or fungistatic used to treat and prevent mycosis such as athlete's foot, ringworm, candidiasis (thrush), serious systemic infections such as cryptococcal meningitis, and others. Such drugs are usually obtained by a doctor's prescription, but a few are available over the counter (OTC). The evolution of antifungal resistance is a growing threat to health globally.

Nystatin, sold under the brand name Mycostatin among others, is an antifungal medication. It is used to treat Candida infections of the skin including diaper rash, thrush, esophageal candidiasis, and vaginal yeast infections. It may also be used to prevent candidiasis in those who are at high risk. Nystatin may be used by mouth, in the vagina, or applied to the skin.

Amphotericin B is an antifungal medication used for serious fungal infections and leishmaniasis. The fungal infections it is used to treat include mucormycosis, aspergillosis, blastomycosis, candidiasis, coccidioidomycosis, and cryptococcosis. For certain infections it is given with flucytosine. It is typically given intravenously.

Fluconazole is an antifungal medication used for a number of fungal infections. This includes candidiasis, blastomycosis, coccidioidomycosis, cryptococcosis, histoplasmosis, dermatophytosis, and tinea versicolor. It is also used to prevent candidiasis in those who are at high risk such as following organ transplantation, low birth weight babies, and those with low blood neutrophil counts. It is given either by mouth or by injection into a vein.

Ergosterol (ergosta-5,7,22-trien-3β-ol) is a mycosterol found in cell membranes of fungi and protozoa, serving many of the same functions that cholesterol serves in animal cells. Because many fungi and protozoa cannot survive without ergosterol, the enzymes that synthesize it have become important targets for drug discovery. In human nutrition, ergosterol is a provitamin form of vitamin D2; exposure to ultraviolet (UV) light causes a chemical reaction that produces vitamin D2.
An antimicrobial is an agent that kills microorganisms (microbicide) or stops their growth. Antimicrobial medicines can be grouped according to the microorganisms they act primarily against. For example, antibiotics are used against bacteria, and antifungals are used against fungi. They can also be classified according to their function. Antimicrobial medicines to treat infection are known as ⠀⠀antimicrobial chemotherapy, while antimicrobial drugs are used to prevent infection, which known as antimicrobial prophylaxis.

In chemistry, an ionophore is a chemical species that reversibly binds ions. Many ionophores are lipid-soluble entities that transport ions across the cell membrane. Ionophores catalyze ion transport across hydrophobic membranes, such as liquid polymeric membranes or lipid bilayers found in the living cells or synthetic vesicles (liposomes). Structurally, an ionophore contains a hydrophilic center and a hydrophobic portion that interacts with the membrane.

Tiabendazole, also known as thiabendazole or TBZ and the trade names Mintezol, Tresaderm, and Arbotect, is a preservative, an antifungal agent, and an antiparasitic agent.
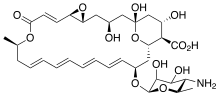
Polyene antimycotics, sometimes referred to as polyene antibiotics, are a class of antimicrobial polyene compounds that target fungi. These polyene antimycotics are typically obtained from certain species of Streptomyces bacteria. Previously, polyenes were thought to bind to ergosterol in the fungal cell membrane, weakening it and causing leakage of K+ and Na+ ions, which could contribute to fungal cell death. However, more detailed studies of polyene molecular properties have challenged this model suggesting that polyenes instead bind and extract ergosterol directly from the cellular membrane thus disrupting the many cellular functions ergosterols perform. Amphotericin B, nystatin, and natamycin are examples of polyene antimycotics. They are a subgroup of macrolides.

Lactobacillales are an order of gram-positive, low-GC, acid-tolerant, generally nonsporulating, nonrespiring, either rod-shaped (bacilli) or spherical (cocci) bacteria that share common metabolic and physiological characteristics. These bacteria, usually found in decomposing plants and milk products, produce lactic acid as the major metabolic end product of carbohydrate fermentation, giving them the common name lactic acid bacteria (LAB).

Butenafine, sold under the brand names Lotrimin Ultra, Mentax, and Butop, is a synthetic benzylamine derived antifungal drug.
A lipopeptide is a molecule consisting of a lipid connected to a peptide. They are able to self-assemble into different structures. Many bacteria produce these molecules as a part of their metabolism, especially those of the genus Bacillus, Pseudomonas and Streptomyces. Certain lipopeptides are used as antibiotics. Due to the structural and molecular properties such as the fatty acid chain, it poses the effect of weakening the cell function or destroying the cell. Other lipopeptides are toll-like receptor agonists. Certain lipopeptides can have strong antifungal and hemolytic activities. It has been demonstrated that their activity is generally linked to interactions with the plasma membrane, and sterol components of the plasma membrane could play a major role in this interaction. It is a general trend that adding a lipid group of a certain length to a lipopeptide will increase its bactericidal activity. Lipopeptides with a higher amount of carbon atoms, for example 14 or 16, in its lipid tail will typically have antibacterial activity as well as anti-fungal activity. Therefore, an increase in the alkyl chain can make lipopeptides soluble in water. As well, it opens the cell membrane of the bacteria, so antimicrobial activity can take place.

Polylysine refers to several types of lysine homopolymers, which may differ from each other in terms of stereochemistry and link position (α/ε). Of these types, only ε-poly-L-lysine is produced naturally.

Clavams are a class of β-lactam antibiotics. These antibiotics are derived from Streptomyces clavuligerus NRRL 3585. This class is divided into the clavulanic acid class and the 5S clavams class. Both groups are the outcomes of the fermentation process produced by Streptomyces spp. Clavulanic acid is a broad-spectrum antibiotic and 5S clavams may have anti-fungal properties. They are similar to penams, but with an oxygen substituted for the sulfur. Thus, they are also known as oxapenams.

Streptomyces natalensis is a bacterial species in the genus Streptomyces.
Streptomyces isolates have yielded the majority of human, animal, and agricultural antibiotics, as well as a number of fundamental chemotherapy medicines. Streptomyces is the largest antibiotic-producing genus of Actinomycetota, producing chemotherapy, antibacterial, antifungal, antiparasitic drugs, and immunosuppressants. Streptomyces isolates are typically initiated with the aerial hyphal formation from the mycelium.
Streptomyces chattanoogensis is a bacterium species from the genus of Streptomyces which has been isolated from soil in Tennessee in the United States. Streptomyces chattanoogensis produces natamycin.
Streptomyces lydicus is a bacterium species from the genus of Streptomyces which has been isolated from soil in the United States. Streptomyces lydicus produces actithiazic acid, natamycin, lydimycin, streptolydigin, and 1-deoxygalactonojirimycin. Streptomyces lydicus can be used as an agent against fungal plant pathogens like Fusarium, Pythium, Phytophthora, Rhizoctonia and Verticillum.
Topical antifungaldrugs are used to treat fungal infections on the skin, scalp, nails, vagina or inside the mouth. These medications come as creams, gels, lotions, ointments, powders, shampoos, tinctures and sprays. Most antifungal drugs induce fungal cell death by destroying the cell wall of the fungus. These drugs inhibit the production of ergosterol, which is a fundamental component of the fungal cell membrane and wall.