
Gram-negative bacteria are bacteria that, unlike gram-positive bacteria, do not retain the crystal violet stain used in the Gram staining method of bacterial differentiation. Their defining characteristic is that their cell envelope consists of a thin peptidoglycan cell wall sandwiched between an inner (cytoplasmic) membrane and an outer membrane. These bacteria are found in all environments that support life on Earth.

Conjunctivitis, also known as pink eye or Madras eye, is inflammation of the conjunctiva and the inner surface of the eyelid. It makes the eye appear pink or reddish. Pain, burning, scratchiness, or itchiness may occur. The affected eye may have increased tears or be "stuck shut" in the morning. Swelling of the sclera may also occur. Itching is more common in cases due to allergies. Conjunctivitis can affect one or both eyes.

Neomycin is an aminoglycoside antibiotic that displays bactericidal activity against Gram-negative aerobic bacilli and some anaerobic bacilli where resistance has not yet arisen. It is generally not effective against Gram-positive bacilli and anaerobic Gram-negative bacilli. Neomycin comes in oral and topical formulations, including creams, ointments, and eyedrops. Neomycin belongs to the aminoglycoside class of antibiotics that contain two or more amino sugars connected by glycosidic bonds.
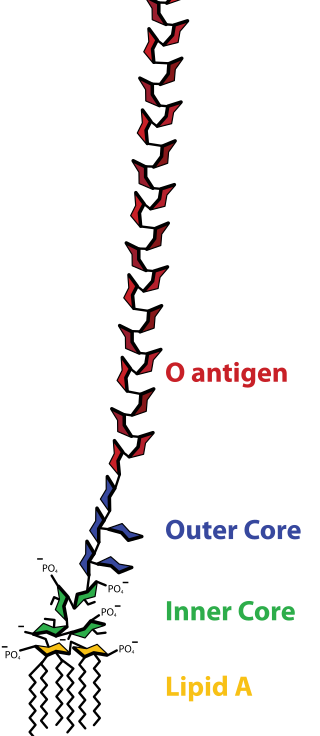
Lipopolysaccharide, now more commonly known as endotoxin, is a collective term for components of the outermost membrane of the cell envelope of gram-negative bacteria, such as E. coli and Salmonella with a common structural architecture. Lipopolysaccharides (LPS) are large molecules consisting of three parts: an outer core polysaccharide termed the O-antigen, an inner core oligosaccharide and Lipid A, all covalently linked. In current terminology, the term endotoxin is often used synonymously with LPS, although there are a few endotoxins that are not related to LPS, such as the so-called delta endotoxin proteins produced by Bacillus thuringiensis.
Aminoglycoside is a medicinal and bacteriologic category of traditional Gram-negative antibacterial medications that inhibit protein synthesis and contain as a portion of the molecule an amino-modified glycoside (sugar). The term can also refer more generally to any organic molecule that contains amino sugar substructures. Aminoglycoside antibiotics display bactericidal activity against Gram-negative aerobes and some anaerobic bacilli where resistance has not yet arisen but generally not against Gram-positive and anaerobic Gram-negative bacteria.

Polymyxins are antibiotics. Polymyxins B and E are used in the treatment of Gram-negative bacterial infections. They work mostly by breaking up the bacterial cell membrane. They are part of a broader class of molecules called nonribosomal peptides.

Colistin, also known as polymyxin E, is an antibiotic medication used as a last-resort treatment for multidrug-resistant Gram-negative infections including pneumonia. These may involve bacteria such as Pseudomonas aeruginosa, Klebsiella pneumoniae, or Acinetobacter. It comes in two forms: colistimethate sodium can be injected into a vein, injected into a muscle, or inhaled, and colistin sulfate is mainly applied to the skin or taken by mouth. Colistimethate sodium is a prodrug; it is produced by the reaction of colistin with formaldehyde and sodium bisulfite, which leads to the addition of a sulfomethyl group to the primary amines of colistin. Colistimethate sodium is less toxic than colistin when administered parenterally. In aqueous solutions, it undergoes hydrolysis to form a complex mixture of partially sulfomethylated derivatives, as well as colistin. Resistance to colistin began to appear as of 2015.
Bacitracin is a polypeptide antibiotic. It is a mixture of related cyclic peptides produced by Bacillus licheniformis bacteria, that was first isolated from the variety "Tracy I" in 1945. These peptides disrupt Gram-positive bacteria by interfering with cell wall and peptidoglycan synthesis.

Haemophilus influenzae is a Gram-negative, non-motile, coccobacillary, facultatively anaerobic, capnophilic pathogenic bacterium of the family Pasteurellaceae. The bacteria are mesophilic and grow best at temperatures between 35 and 37 °C.

Cefixime, sold under the brand name Suprax among others, is an antibiotic medication used to treat a number of bacterial infections. These infections include otitis media, strep throat, pneumonia, urinary tract infections, gonorrhea, and Lyme disease. For gonorrhea typically only one dose is required. In the United States it is a second-line treatment to ceftriaxone for gonorrhea. It is taken by mouth.

Gramicidin, also called gramicidin D, is a mix of ionophoric antibiotics, gramicidin A, B and C, which make up about 80%, 5%, and 15% of the mix, respectively. Each has 2 isoforms, so the mix has 6 different types of gramicidin molecules. They can be extracted from Brevibacillus brevis soil bacteria. Gramicidins are linear peptides with 15 amino acids. This is in contrast to unrelated gramicidin S, which is a cyclic peptide.

Neomycin/polymyxin B/bacitracin, also known as triple antibiotic ointment, is an antibiotic medication used to reduce the risk of infections following minor skin injuries. It contains the three antibiotics neomycin, polymyxin B, and bacitracin. It is for topical use.

Tobramycin is an aminoglycoside antibiotic derived from Streptomyces tenebrarius that is used to treat various types of bacterial infections, particularly Gram-negative infections. It is especially effective against species of Pseudomonas.

Cefotaxime is an antibiotic used to treat several bacterial infections in humans, other animals, and plant tissue culture. Specifically in humans it is used to treat joint infections, pelvic inflammatory disease, meningitis, pneumonia, urinary tract infections, sepsis, gonorrhea, and cellulitis. It is given either by injection into a vein or muscle.

N-Acetylneuraminic acid is the predominant sialic acid found in human cells, and many mammalian cells. Other forms, such as N-Glycolylneuraminic acid, may also occur in cells.

Pathogenic bacteria are bacteria that can cause disease. This article focuses on the bacteria that are pathogenic to humans. Most species of bacteria are harmless and many are beneficial but others can cause infectious diseases. The number of these pathogenic species in humans is estimated to be fewer than a hundred. By contrast, several thousand species are part of the gut flora present in the digestive tract.

Polypeptide antibiotics are a chemically diverse class of anti-infective and antitumor antibiotics containing non-protein polypeptide chains. Examples of this class include actinomycin, bacitracin, colistin, and polymyxin B. Actinomycin-D has found use in cancer chemotherapy. Most other polypeptide antibiotics are too toxic for systemic administration, but can safely be administered topically to the skin as an antiseptic for shallow cuts and abrasions.

Meningitis is acute or chronic inflammation of the protective membranes covering the brain and spinal cord, collectively called the meninges. The most common symptoms are fever, intense headache, vomiting and neck stiffness and occasionally photophobia. Other symptoms include confusion or altered consciousness, nausea, and an inability to tolerate light or loud noises. Young children often exhibit only nonspecific symptoms, such as irritability, drowsiness, or poor feeding. A non-blanching rash may also be present.
Haemophilus meningitis is a form of bacterial meningitis caused by the Haemophilus influenzae bacteria. It is usually associated with Haemophilus influenzae type b. Meningitis involves the inflammation of the protective membranes that cover the brain and spinal cord. Haemophilus meningitis is characterized by symptoms including fever, nausea, sensitivity to light, headaches, stiff neck, anorexia, and seizures. Haemophilus meningitis can be deadly, but antibiotics are effective in treating the infection, especially when cases are caught early enough that the inflammation has not done a great deal of damage. Before the introduction of the Hib vaccine in 1985, Haemophilus meningitis was the leading cause of bacterial meningitis in children under the age of five. However, since the creation of the Hib vaccine, only two in every 100,000 children contract this type of meningitis. Five to ten percent of cases can be fatal, although the average mortality rate in developing nations is seventeen percent, mostly due to lack of access to vaccination as well as lack of access to medical care needed to combat the meningitis.


















